Reports: DNI354178-DNI3: Preparation of Low-Coordinate Bis(Alkoxide) Metal Complexes and Their Reactivity in Bond Formation and Bond Activation Reactions Involving Azide Precursors
Stanislav Groysman, Wayne State University
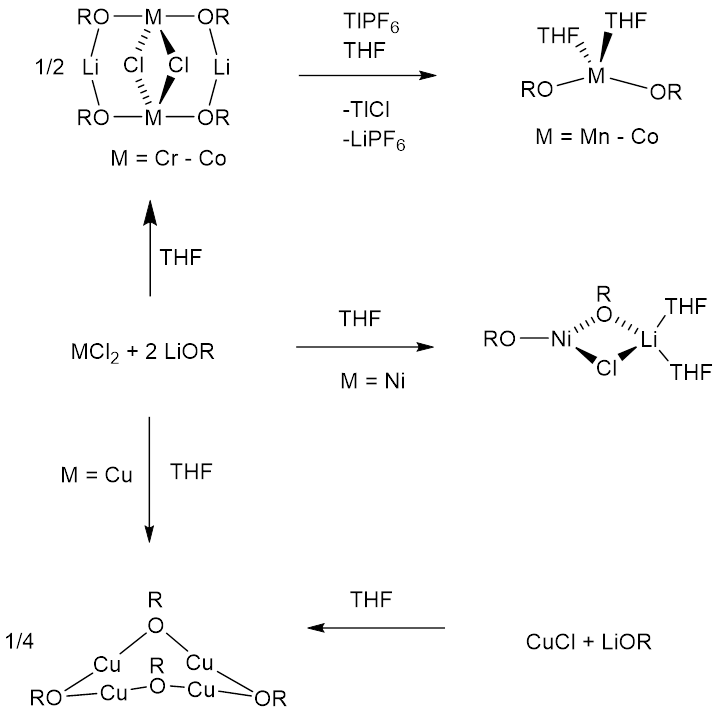
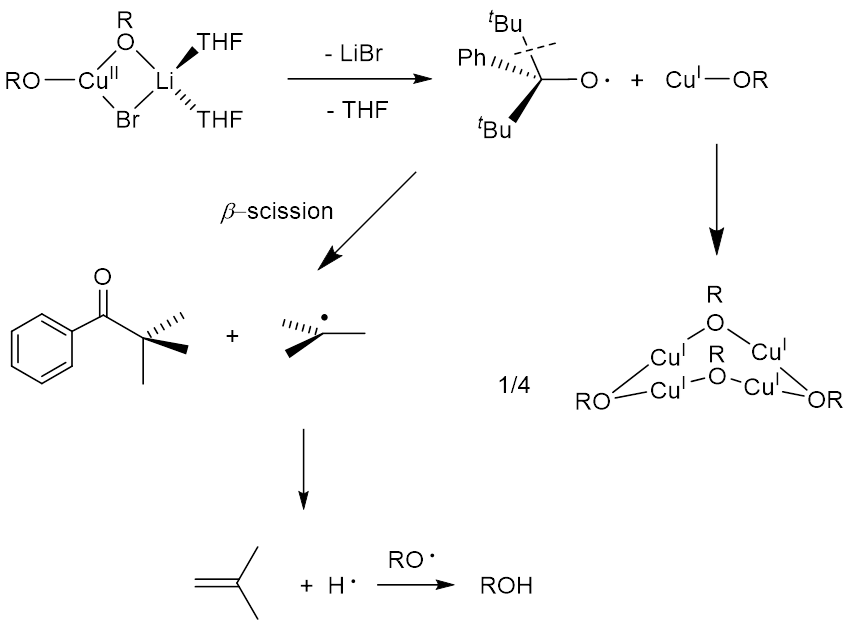
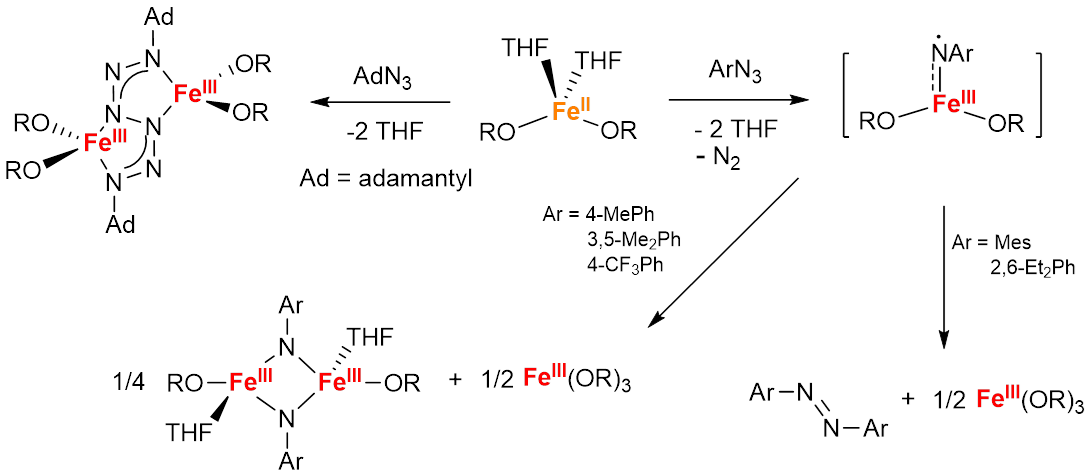
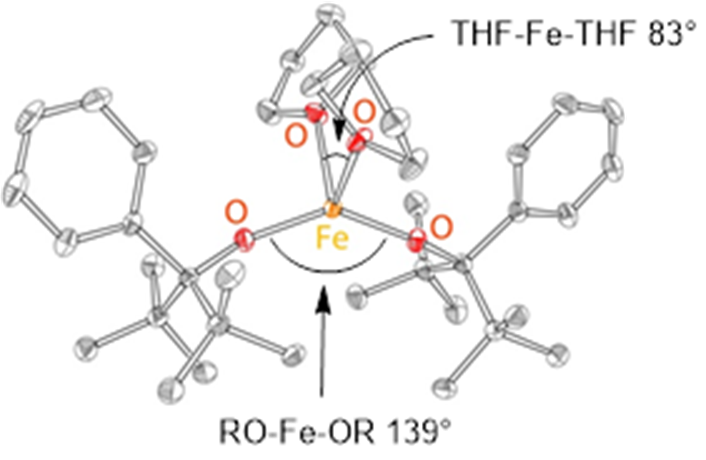 |
Stanislav Groysman, Wayne State University


 |

Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society