Reports: ND353349-ND3: Well-Defined Zinc and Aluminum Catalysts for Reduction of Petroleum Derived Products
Georgii I. Nikonov, PhD, Brock University
We have achieved significant progress in both research directions outlined in the original proposal.
1) Activation of challenging bonds on Al(I) center
We have previously shown that
the Al(I) compound NacNacAl (1, NacNac = [ArNC(Me)CHC(Me)NAr]−
and Ar = 2,6-Pri2C6H3) can easily add H-X (X = H, Si, B, Al, C, N, P, O) s bonds, thus mimicking the key
bond activation step in transition metal catalysis.[1] We
have now extended these oxidative additions to the activation of particularly
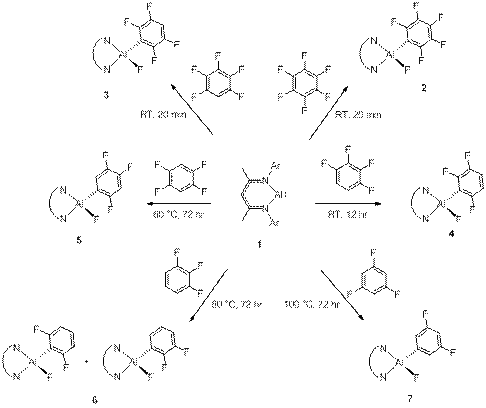
challenging bonds, such as C-O and C-F. Thus, hexafluorobenzene reacts
with 1 occurs already at room temperature and affords NacNacAlF(C6F5)
(2) in only 20 minutes. Pentafluorobenzene also adds to
give regiospecifically the aryl fluoride NacNacAlF(3-C6F4)
(3), with no evidence of compromising C-H bond addition. The 1,2,3,4
isomer of tetrafluorobenzene requires a much longer reaction time (16 h) but
only the product of insertion into the 2-position is formed. This
regioselectivity is controlled by the increasing number of ortho C-F
groups in the product which strengthens the Al-C bond formed. In contrast, addition
of 1,2,4,5-C6H2F4 occurs only at elevated
temperature (60° C) to give exclusively
the product of C-F activation, NacNacAlF(5-C6H2F3-1,2,4)
(5). Further decrease of the number of fluorine atoms in the starting
arene increases the reaction temperature significantly (Scheme 1). Prolonged
heating of 1 with fluorobenzene (days at 100 °C) resulted in decomposition without formation of any aluminum
fluoride species. The products of C-F bond oxidative addition were completely
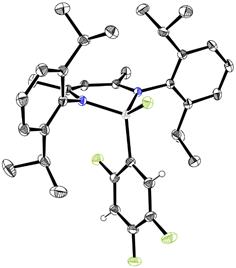
characterized by NMR spectroscopy and by X-ray diffraction of 5 (Figure
1).
Scheme 1. Oxidative addition of C-F bond in polyfluorinated aromatics to 1
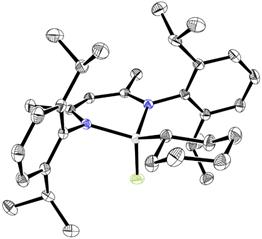
Moreover, we found that these addition reactions can be extended to the activation of the more challenging C(sp3)-F bond. Thus, 1-fluorohexane adds to compound 1 readily at room temperature affording the fluoride derivative NacNacAlF(C6H13) (8). Oxidative addition of the secondary C-F bond of fluorocyclohexane also takes place under very mild conditions to give the aluminum fluoride NacNacAlF(C6H11) (9). Remarkably, NMR monitoring revealed that these additions take place even at temperatures down to −60 °C! The formations of compounds 8 and 9 represent the first examples of alkyl-fluorine bond oxidative addition to any metal center. The structures of these products were studied by NMR spectroscopy and X-ray diffraction analyses (Figure 2).
Figure 1. Molecular structure of 5.
Figure 2. Molecular structure of 9.
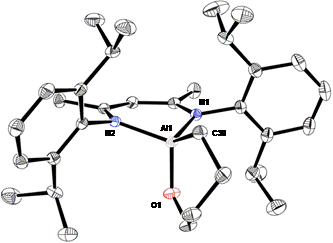
The C-O bond (358 kJ/mol) can be similarly activated. Initial experiments with diethyl ether showed no reaction. But the less bulky substrate tetrahydrofuran adds to 1 at room temperature to give the cyclic alkoxide derivative NacNacAl(-OCH2CH2CH2CH2-) (10, Figure 3).
Figure 3. Molecular structure of 10.
Kinetic studies on the addition of 1,2,3,4-tetrafluorobenzene to 1 established the first order dependence in the aluminum compound and the first order dependence in the reagent as well. The overall reaction is therefore second order, consistent with a simple one-step C-F oxidative addition process. The large negative entropy of activation, ΔS≠ = −108.9(3) J/K.mol, points to a highly ordered transition state, supporting the concerted oxidative addition mechanism.
2) Preparation of mononuclear Zn(I) and the first Zn(0) compounds.
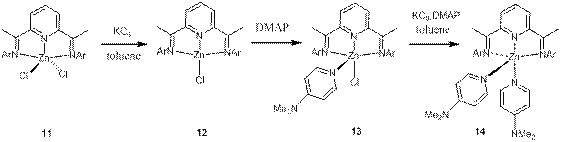
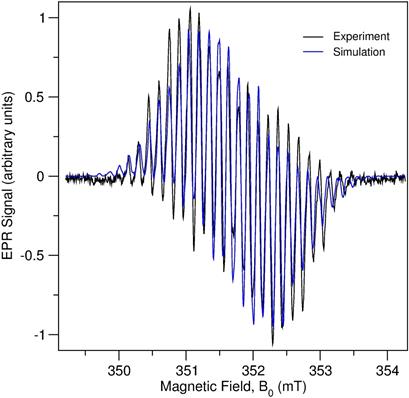
We have previously reported a Ge(0) compound stabilized by the non-innocent diiminopyridine (dimpyr) ligand.[2] We have now extended this chemistry to the Zn(I) and Zn(0) dimpyr complexes. The reaction of compound dimpyrZnCl2 (11) with an equivalent of KC8 in benzene afforded the neutral complex dimpyrZnCl (12) in the formal Zn(I) oxidation state (Scheme 2). Similar reduction of 2 in the presence of DMAP (4-dimethylaminopyridine) results in the Zn(I) derivative dimpyrZnCl(DMPA) (4). EPR spectra 3 and 4 (Figure 4) are consistent with the presence of a single electron delocalized over the dimpyr ligand.
Scheme 2. Preparation of compounds 12, 13, and 14. Ar=2,6-iPr2C6H3, DMAP = 4-dimethylaminopyridine
Figure 4. EPR spectrum of compound 12
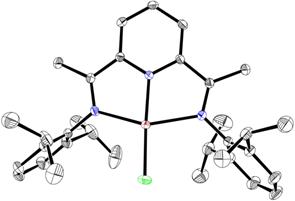
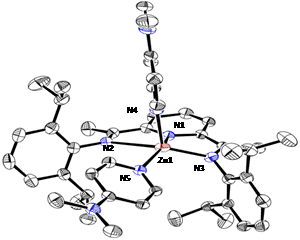
Further reduction of compound 13 in the presence of another equivalent of DMAP results in the formal Zn(0) species dimpyrZn(DMAP)2 (14) (Scheme 2). The structures of complexes 12, 13 and 14 were established by X-ray diffraction analysis. The molecular structure of complex 12 (Figure 5) shows a nearly planar geometry around the zinc center (the mean deviation from the Zn1-N1-N2-N3-Cl1 least square plane is 0.146 Å). Such a flat coordination environment is very unusual for a four-coordinate zinc center and finds the only precedents in zinc porphyrines.
Figure 5. Molecular structure of 12.
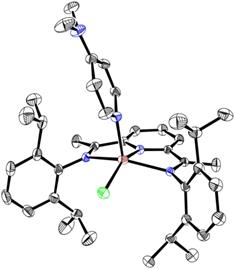
The coordination of DMAP to the zinc(I) center results in a five-coordinate, square base pyramidal geometry of 13 featuring elongated bonds around zinc (Figure 2, right). Thus, the Zn(1)-N1 distance to the central pyridine ring elongates from 1.9400(16) Å in 12 to 1.9967(18) in 13 and the Zn(1)-Cl(1) distance elongates from 2.1566(6) to 2.2255(7) Å, respectively. The Zn-imine distances at 2.2459(19) and 2.3262(19) Å are also noticeably longer than the corresponding bonds in 12. The C=N(imine) distances, however, remain virtually undisturbed (1.294(3) and 1.304(3) Å).
Figure 6. Molecular structure of 13.
X-ray structure determination from a single crystal of 14 revealed the presence of two DMAP ligands, one trans to the central pyridine and the other approximately orthogonal to the dimpyr plane (Figure 7). In contrast to the parent compounds 12 and 13, the zinc atom occupies a very asymmetric position, with one Zn-imine distance being very short at 2.0265(14) Å and the other one being very long (2.585 (1) Å), so that the zinc atom adopts an unprecedented see-saw geometry. The asymmetric coordination of zinc results in more pronounced differences between two imine fragments. Thus, the imine moiety only loosely coordinated to zinc has the C=N bond of 1.3005(18) Å, whereas the tightly coordinated arm shows a much longer C-N distance of 1.3893(16) Å. The latter bond is very close to the N-aryl distance of 1.4096(19) Å and is consistent with a nearly complete reduction of the C=N double bond.
Figure 7. Molecular structure of 14.
span>[1] T. Chu, I. Korobkov, G.I. Nikonov, J. Am. Chem. Soc. 2014, 136, 9195−9202.
span>[2] T. Chu, L. Belding, A. van der Est, T. Dudding, I. Korobkov, G.I. Nikonov, Angew. Chem. Int. Ed. 2014, 53, 2711 – 2715.