Reports: DNI1052827-DNI10: Elucidating the Electronic Structure and Chemistry of Layered Vanadium Oxides for Next-Generation Energy Storage
Louis Piper, PhD, Binghamton University
Our ACS PRF funded project has focused on the evolution of the electronic structure of promising materials for next generation Li-ion battery cathodes. Recently, nano-engineered LiFePO4 olivine has replaced costly and thermally unstable LiCoO2. The use of polyanions adversely reduces the specific capacity; one way to compensate for the loss of capacity is to develop a material containing a polyatomic anion (thermally stable) that is capable of inserting two lithium (or sodium) atoms per redox-active metal ion, such as VOPO4. In general one would extend this to a system that is already thermal stable and environmentally benign that can accommodate more two or more lithium (or sodium) per redox-active metal, e.g. V2O5•nH2O aerogels (or delta-phase V4O10•nH2O).
Our research has focused on addressing the ultimate question: what is happening at the microscopic level when more than 2 Li per vanadium are intercalated into V2O5•nH2O aerogels? In our second year report we summarize our x-ray photoemission spectroscopy (XPS) and pairs distribution function (PDF) measurements of V2O5•nH2O aerogels as a function of electrochemical lithiation.
Results:
1) Role of tightly bound water species:
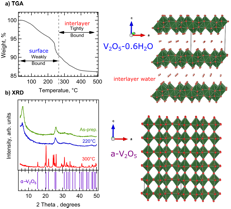
The presence of water has been shown to play an important role in determining the interlayer distance and electrochemical performance of V2O5•nH2O aerogels. Surface water and adsorbed species on the aerogel can be eliminated by heating the aerogel to 220C without collapsing the sparse aerogel structure. Heating to 300C removes the remaining tightly bound water (i.e. crystalline water species within the interlayers) resulting in the formation of dense crystalline orthorhombic V2O5 (Fig. 1).
Fig. 1 Thermogravimetric analysis (TGA) and x-ray diffraction (XRD) of as-prepared aerogel as a function of heating. Heating to 220C results in V2O5•0.6H2O aerogel, where only the interlayer H2O remains. Heating to 300C results in the crystalline alpha-phase V2O5 phase.
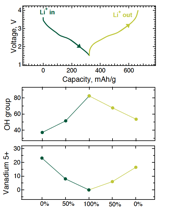
Fig. 2: (Top) The first cycle discharge/curve curve of our V2O5•0.6H2O aerogel cathodes. (Bottom) XPS analysis summarizing the evolution of the V5+ and LiOH species during the first cycle.
To determine how the Li+ reacts with the interlayer tightly bound water species, we examined the evolution of the chemical environment of electrochemically discharged and charged V2O5•nH2O aerogels heat treated to 220°C using XPS. Our studies revealed that in addition to the expected reversible vanadium reduction and oxidation associated with the intercalation process; we observed the partially irreversible formation of LiOH species (Fig. 2). This is attributed to the reaction between Li+ and the tightly bound interlayer water, and the accumulation of inactive Li species during each cycle is considered responsible for the gradual capacity fade observed in our experiments. These studies are currently under review for publication.
2) Local structure of V2O5•nH2O aerogels:
The xerogel structure was solved by Petkov et al. [J. Am. Chem. Soc. 2002] using atomic PDF i.e. the Fourier transform of the total scattering diffraction pattern. This method provides local, medium and long-range structural information because it is sensitive to Bragg and diffuse scattering contributions. This makes it ideal for studying materials with no long-range order, such as sparse aerogel and xerogel forms. The xerogel consists of a double layered structure with water molecules in between them by (as shown in Fig. 1), often referred to as delta-phase V4O10•nH2O. The aerogel structure has not been explicitly solved but it is assumed to be similar to the xerogel structure. Moreover, its evolution of the local structure as a function of lithium content is not known.
We performed PDF experiments at NSLS-II at Brookhaven National Laboratory of out V2O5•0.6H2O aerogel electrodes at various stages of discharge and charge. Our experimental data is currently being analyzed, and we are collaborating with Prof. Watson at Trinity College Dublin who is performing atomistic simulations of the structure. These calculations explicitly consider the weak dispersive forces associated with layered materials.
Additional studies:
Our research on the electronic structure of intercalated V2O5 nanowires. We previously reported upon the nature of the lone pair distortion in Pb2+ intercalated V2O5 nanowires. [L. Wangoh et al., Appl. Phys. Lett. (2014)] We were able to show that the lone pair distortion is facilitated by the hybridization of the Pb 6s with the O 2p orbitals, which raises the valence band maximum (compared to V2O5). The modified band alignment was explored further for water splitting applications. This study is published as Integrating β-Pb0.33V2O5 Nanowires with CdSe Quantum Dots: Toward Nanoscale Heterostructures with Tunable Interfacial Energetic Offsets for Charge Transfer by Kate E. Pelcher, Christopher C. Milleville, Linda Wangoh, Saurabh Chauhan, Matthew R. Crawley, Peter M. Marley, Louis F. J. Piper, David F. Watson, and Sarbajit Banerjee in Chemistry of Materials, vol. 27 pp 2468–2479 (DOI: 10.1021/cm504574h). Other lone pair active ions (Sn2+) will be examined.