Reports: ND353842-ND3: Studies of Magnetic Properties of Metalloporphyrins by Inelastic Neutron Scattering
Ziling (Ben) Xue, PhD, University of Tennessee
Since the start of the project, we have prepared model metalloporphyrins and conducted two inelastic neutron scattering (INS) experiments at Oak Ridge National Laboratory (ORNL). Two articles have been published in Inorganic Chemistry, acknowledging PRF support. A third article, submitted to Inorganic Chemistry, is being revised based on reviewers’ comments. In addition, we have given two ACS National Meeting presentations (Spring 2014, Dallas; Fall 2015, Boston) about the PRF-supported research. Dr. Xue gave an invited talk at the 29th Chinese Chemical Society Congress in August 2014. Three Ph.D. students have been supported by the grant. One, Seth C. Hunter, graduated in Spring 2015 with Ph.D. degree.
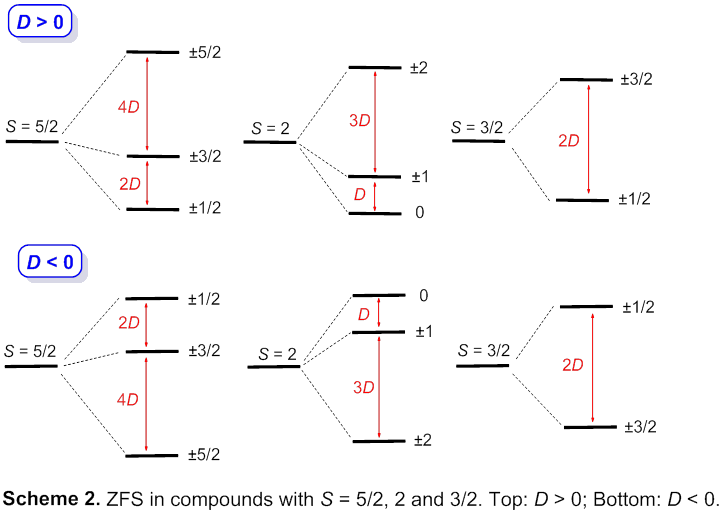
Porphyrins in fossils, also known as geoporphyins, are the most abundant source of porphyrins on the earth. Geochemistry of metal complexes in fossils has been shown to be predominantly that of metalloporphyrins. Metalloporphyrins are often paramagnetic. We have probed metalloporphyrins by INS to obtain their zero-field splitting (ZFS) parameters (D), constants for compounds with S > 1/2. ZFS leads to magnetic anisotropy with profound effects on magnetic properties of the compounds. INS is a method to directly give the D values. To our knowledge, INS has not been used to study metalloporphyrins.
We have focused on [M(TPP)X] (H2TPP = meso-tetraphenylporphyrin; X = halides) and [M(TPP)]. These are model metalloporphyrins with different transition metals, metals at different oxidation states, and compounds with different axial ligands.
I. Our Initial INS Studies
The work was reported in a 2014 paper in Inorganic Chemistry. We determined ZFS parameters D of [Fe(TPP)Cl], [Mn(TPP)Cl], [Cr(TPP)Cl], and [Mn(TPP)]. Molecules of these complexes have 4-fold symmetry (Scheme 1). If their crystals also have a 4-fold symmetry in, e.g., a tetragonal space group, electronic ground states of complexes with S = 5/2, 2, 3/2 and 1 are split, as a result of ZFS (Scheme 2). Two examples are summarized.
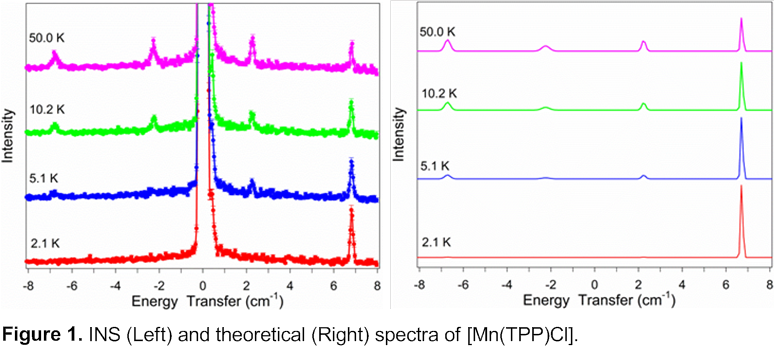
[Mn(TPP)Cl]. Measured and theoretical INS spectra are shown in Figure 1. At 2.1 K there is only one peak at 6.70(3) cm-1. When the temperature is increased, a second peak appears at 2.24(3) cm-1. The fact that only the larger ±2 -> ±1 transition is populated at the low temperature of 2.1 K indicates that D is negative, giving D = -2.24(3) cm-1.
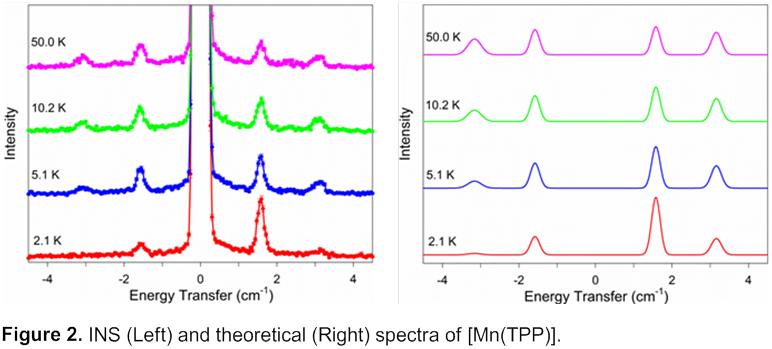
[Mn(TPP)]. To our knowledge, ZFS of [Mn(TPP)] has not been reported. Measured and theoretical INS spectra are shown in Figure 2, which are consistent with a positive D value, giving D = 0.79(2) cm-1.
II. INS and Ab Initio Studies of [Fe(TPP)X] (X = F, Br, I)
The manuscript, after reviews, is in revision for Inorganic Chemistry. The ab initio studies of ZFS, including [Fe(TPP)Cl], are a collaboration with Drs. Mihail Atanasov and Frank Neese of Max Planck Institute , Germany, and Bulgarian Academy of Sciences (Dr. Mihail Atanasov).
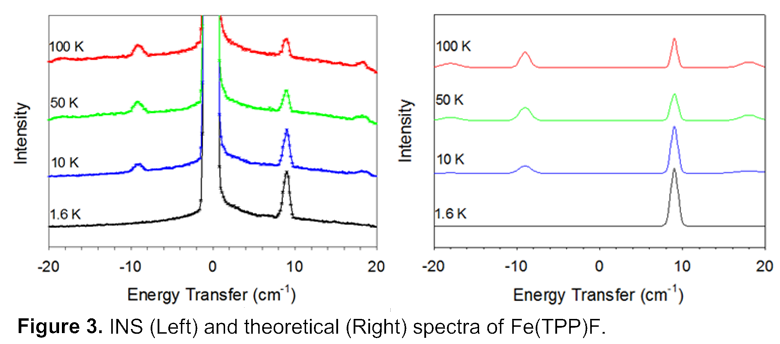
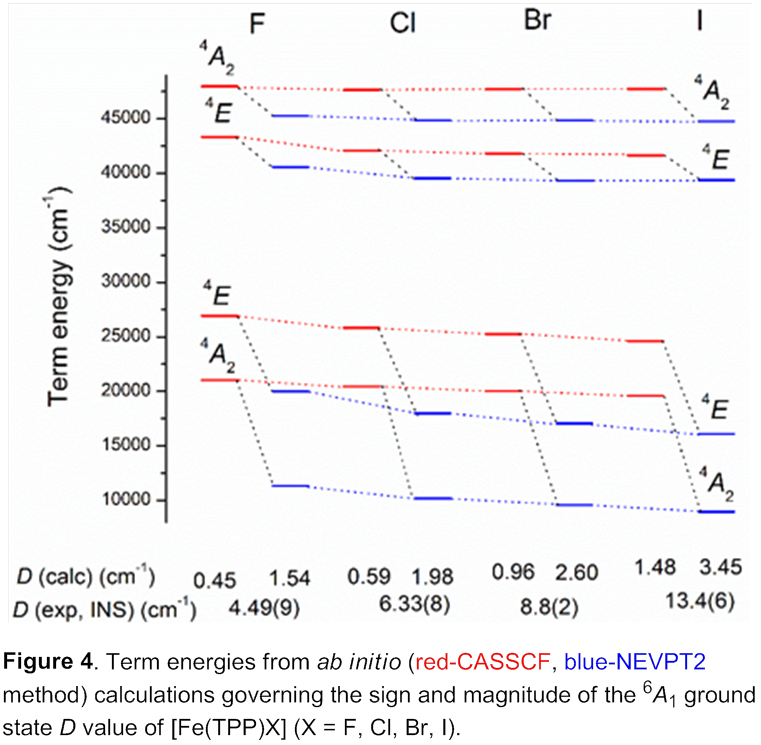
INS work shows the following trend of D values: [Fe(TPP)F] < [Fe(TPP)Cl] < [Fe(TPP)Br] < [Fe(TPP)I]. INS spectra of [Fe(TPP)F] are given in Figure 3. These data provide a rare, complete determination of ZFS parameters in a metalloporphyrin halide series. Their electronic structures have been studied to explore the origin of the large and positive D of their 6A1 ground state.
The increase in the D values is related to the lowering of the energy gap between the 6A1 ground state and the 4T1 lowest excited state (Figure 4). This lowering is attributed to the weakening of both the s and the p antibonding interactions between the Fe3+ ion and the axial halide ligand.
The current work not only reports ZFS parameters by INS but also identifies key factors that determine the sign/magnitude of D values in the single ion compounds.
III. INS Studies of [Mn(TPP)X] (X = F, Br, I) and [V(TPP)Cl]
We have prepared the complexes and obtained their INS data. We are analyzing the data of [Mn(TPP)X] and [V(TPP)Cl].
IV. Intermolecular Interactions in Solid-State Metalloporphyrins and Their Impacts on Crystal and Molecular Structures
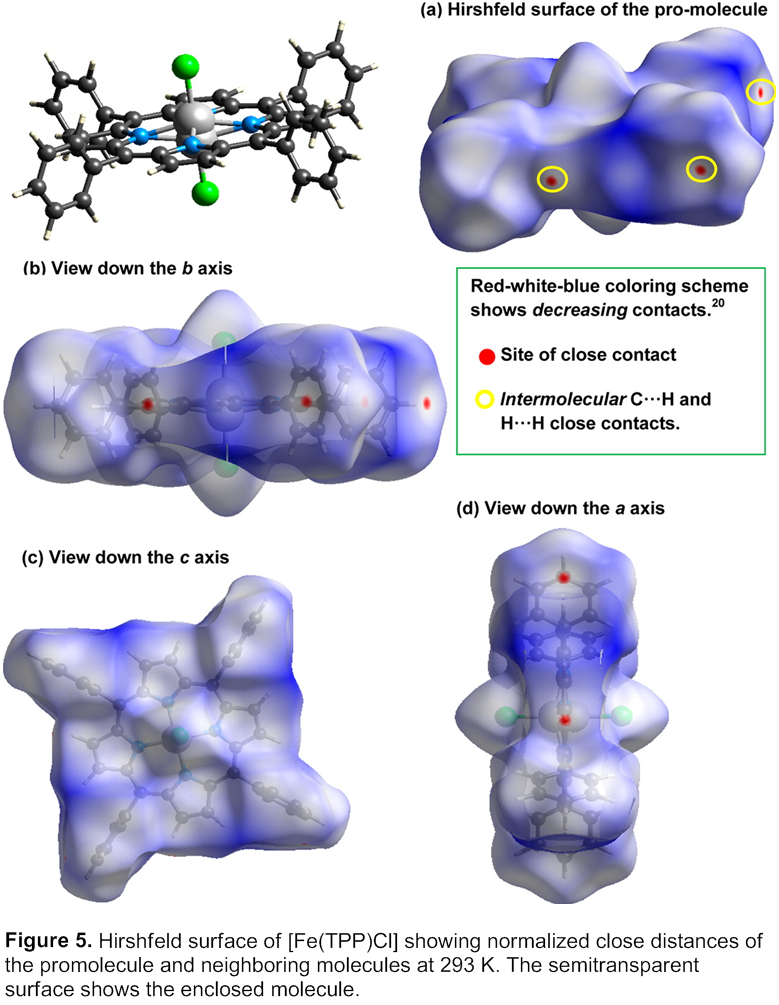
This work was published in 2014 Inorganic Chemistry. INS studies of [Fe(TPP)Cl] require knowledge of its crystal structure at 5 K, which was found to be disordered. Variable-temperature structure study and Hirshfeld surface analyses of its structures and previously published structures of [M(TPP)(NO)] (M = Fe, Co) reveal that intermolecular interactions are a significant factor in structure disorder in the metalloporphyrins and phase changes in the nitrosyl complexes. Hirshfeld analyses of the structure of [Co(TPP)(NO)] indicate that the phase change from I4/m to P-1 leads to an increase in void-volume percentage, permitting additional structural compression through tilting of the phenyl rings to offset the close-packing interactions at the interlayer positions in the crystal structures with temperature decrease. X-ray and neutron structure studies of [Fe(TPP)Cl] at 293, 143 and 20 K reveal a tilting of the phenyl groups away from being perpendicular to the porphyrin ring as a result of intermolecular interactions. The current Hirshfeld surface analyses thus reveal intermolecular interactions in the crystals of [M(TPP)X] (M = Fe, X = Cl; M = Co, Fe, X = NO) lead to phase changes in [M(TPP)(NO)] and a gradual structural change in [Fe(TPP)Cl], allowing reversible thermal compression at lower temperatures.
We are continuing our INS studies of metalloporphyrins.