Reports: ND254850-ND2: Oxygen-17 in Sedimentary and Diagenetic Carbonates
Benjamin Passey, PhD, University of Michigan–Ann Arbor
Stable isotopes in sedimentary carbonates have long been used to infer aspects of the environment in which the carbonate formed, including temperature and water isotopic composition, which itself relates to climate, the degree to which water has been pre-evaporated, and seawater mixing, among other factors. The advent of clumped isotope thermometry in the past decade has allowed the temperature signal to be unambiguously resolved from the water isotopic composition signal. We proposed to develop triple oxygen isotope systematics (17O/16O relative to 18O/16O) of sedimentary carbonates in order to better interpret the nature of paleo-fluids. In particular, evaporation should have a strong influence on the triple oxygen isotope composition of fluids, and seawater should be distinct from meteoric waters.
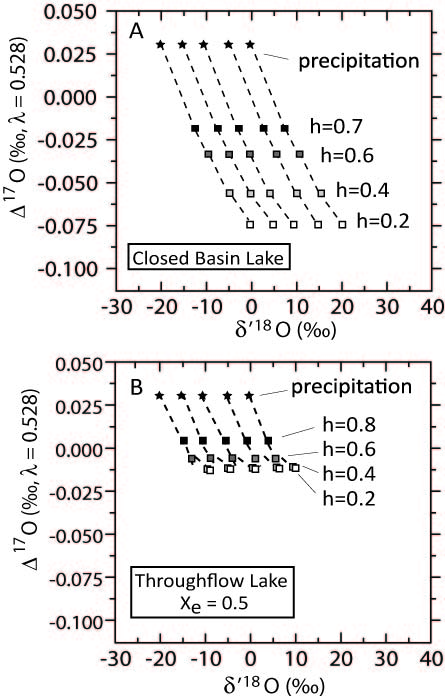
The research has involved four main lines of investigation. In the first, we developed triple oxygen isotope models for closed-basin and flow-through lakes, with parameters including fraction of water leaving the lake by evaporation versus runoff, isotopic composition of ambient water vapor, relative humidity, and parameters related to the diffusive versus advective transport of water vapor away from the water surface. All models are cast in terms of isotope ratios (R-values) and fractionation factors (alpha-values) for maximum precision and usability in the triple oxygen isotope system. The models predict that lake waters that have experienced partial evaporation under conditions of low relative humidity will be marked by a deficiency in 17O. Inasmuch, 17O promises to be a powerful indicator of climate and hydrological conditions in lacustrine systems in the fossil record.
Figure 1. Modeled Triple oxygen isotope compositions of lake waters for (A) closed basin lakes, and (B) flow-through lakes where half of the water leaves by evaporation, and the remainder leaves as flow-through or groundwater. The y-axis is the deviation in 17O/16O from a reference relationship with 18O/16O, and the x-axis is the 18O/16O value of the sample relative to VSMOW, cast in "delta-prime" notation. h denotes relative humidity, and Xe denotes fraction of water leaving the lake system by evaporation.
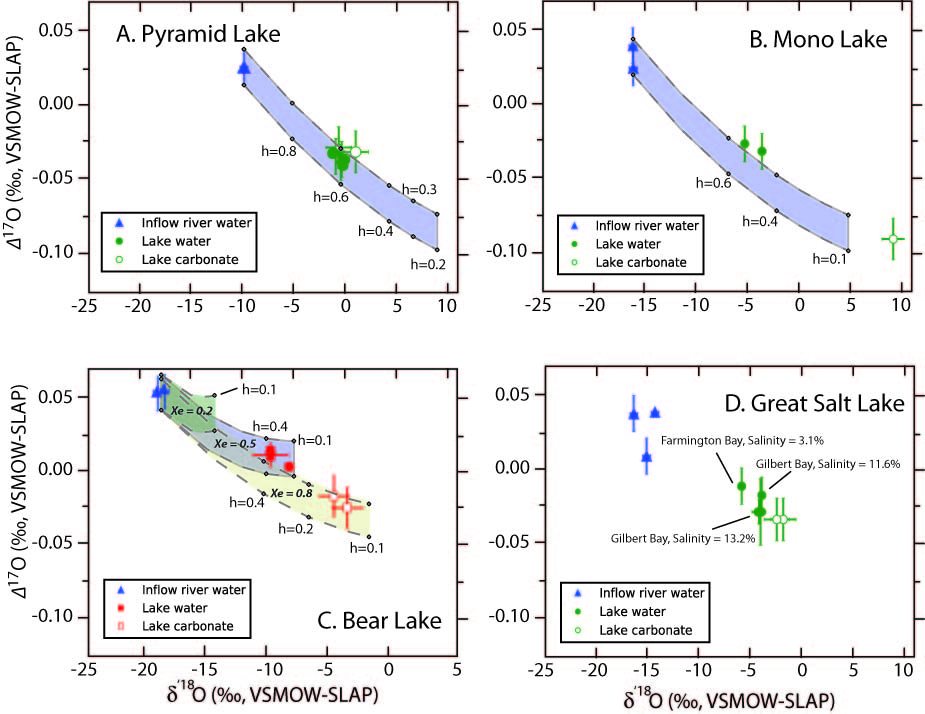
For the second line of investigation, collected and analyzed lake waters, lake carbonates, and input river waters for several lakes in the western United States (Bear Lake, UT/ID; Great Salt Lake, UT; Mono Lake, CA; Pyramid Lake CA/NV; and Lake Tahoe, CA), and compared the measured isotopic compositions to our model predictions. There is generally good agreement between models and data, and the data bear out the prediction that evaporated waters contain a marked deficiency in 17O compared to unevaporated waters.
Figure 2. Measured triple oxygen isotope compositions of lake waters and lake carbonates (the latter given as equivalent water values) for (A) Pyramid Lake, (B) Mono Lake, (C) Bear Lake, and (D) Great Salt Lake. Shaded regions represent model predictions for given ranges of relative humidity (h), and fraction evaporative water loss from the lake (Xe).
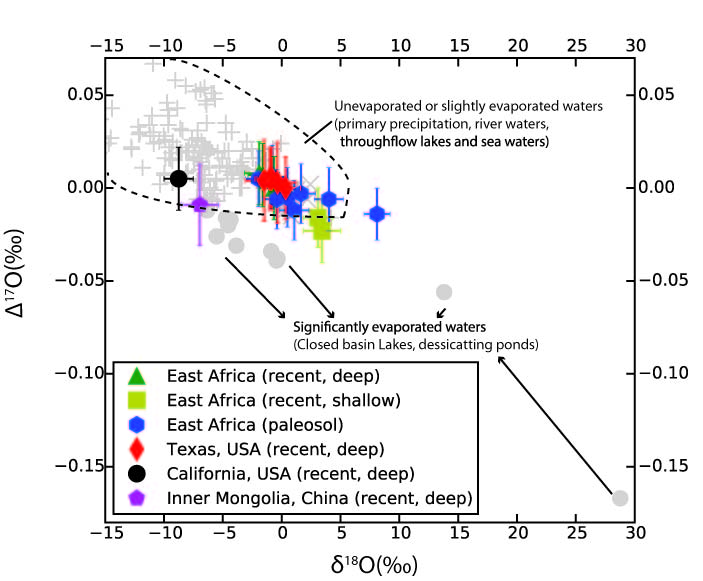
In a third line of investigation, we created a numerical model of triple oxygen isotopes in soil water, and measured a representative sample of modern soil carbonate samples from around the world to gain a first-order sense of the triple oxygen isotope systematics of soil carbonates. Oxygen-17 deficits in soil systems are present, but modest compared to deficits in lake systems. Nevertheless, the signal is resolvable, and analysis of triple oxygen isotopes in soil carbonates promises to reveal valuable information about local climate and aridity during the time of carbonate growth.
Figure 3. Triple oxygen isotope compositions of recent and fossil soil carbonates (expressed as equivalent soil water; colored symbols) compared to compositions of precipitation (light gray crosses) and evaporated lakes (gray circles).
A final line of investigation focused on triple oxygen isotope fractionation exponents between water and carbonate at temperatures ranging from 5 – 60 °C. These values are based on careful experiments where calcium carbonate was grown in the laboratory under controlled temperatures using a passive CO2-degassing approach. The triple oxygen isotope compositions of the water and carbonate were determined (the latter as CO2 evolved from acid digestion of carbonate) and used to calculate the triple isotope fractionation exponent for each growth temperature. We also calibrated the clumped isotope thermometer using the same samples. As predicted by theory, we observed minimal temperature-dependency of the triple isotope fractionation exponent in this temperature range.
Much of the above formed the basis of the PhD dissertation of Dr. Haoyuan Ji (PhD October 2016), and also involved effort and training of three additional PhD students and one undergraduate student at Johns Hopkins: Dr. Huanting Hu (PhD September 2016); Dr. Sophie Lehmann (PhD September 2017); Ms. Dana Brenner (PhD expected Spring 2018), and Mr. Tucker Gordon (B.S. Spring 2016).