Reports: DNI754951-DNI7: Reaction Kinetic Elucidation and Gas Transport Characterization of Interfacially Photopolymerized, Intrinsically Microporous Membranes
Timothy F. Scott, PhD, University of Michigan
This project involves the improvement of membrane-based gas separation efficiency, a challenge that remains a formidable owing to the compromise between attaining high selectivity and high permeability. Specifically, the reaction kinetics of interfacial copper (I)-catalyzed azide–alkyne cycloaddition (CuAAC) photopolymerizations using rigid monomeric precursors and an examination of the resultant (micro)porous organic polymer thin film structure and gas separation characteristics are examined.
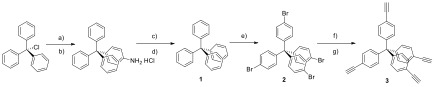
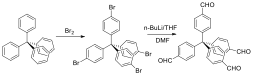
Initial work involved the synthesis of rigid, tetra-functional monomers from commercially-available precursors. From the precursor triphenylmethylchloride, tetrakis(4-bromophenyl)methane was synthesized in three steps (Scheme 1, compound 2) at an overall yield of 79%. This compound was subject to a Sonogashira coupling with trimethylsilylacetylene which, upon cleavage of the silyl protecting group with tetrabutylammonium fluoride (TBAF), yielded tetrakis(4-ethynylphenyl)methane (Figure 1, compound 3, 80% yield over two steps) as a rigid, tetraalkyne-bearing monomer for CuAAC photopolymerization with rigid, multi-functional azide-bearing monomers, work that is currently ongoing.
Figure 1. Synthesis of tetrakis(4-ethynylphenyl)methane (3). Reagents and conditions: (a) aniline, 190°C, (b) reflux with 2 N HCl/MeOH, (c) H2SO4/EtOH, isoamylnitrite, -10°C, (d) reflux with hypophosphorous acid, (e) Br2, (f) trimethylsilylacetylene, diisopropylamine, triphenylphosphine, PdCl2(PPh3)2, CuI, 80°C, (g) TBAF, benzene/MeCN.
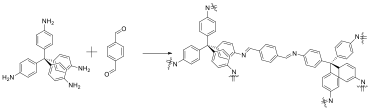
Owing to the irreversibility of the CuAAC reaction, the cross-linked, (micro)porous organic polymer (MOP) films generated by reaction of tetrakis(4-ethynylphenyl)methane with azide-bearing monomers are amorphous with varying pore dimensions. Thus, to determine the influence of pore size dispersity, covalent organic framework (COF) polymers, crystalline cross-linked materials fabricated using dynamic covalent bond-forming reactions, have been synthesized and characterized using monomers analogous to those used for the MOP films. Here, tetraphenylmethane and tetrakis(4-bromophenyl)methane (synthesized as described above) are used as precursors to afford the tetra-functional, amine- and aldehyde-bearing monomers tetrakis(4-aminophenyl)methane (Figure 2a) and tetrakis(4-formylphenyl)methane (Figure 2b), respectively. Subsequently, COFs were synthesized from tetrakis(4-aminophenyl)methane and terephthaldehyde using solvothermal conditions at raised temperature (Figure 3) and using Sc(III)OTf as a Lewis acid catalyst for imine metathesis at room temperature (see Table 1).
Figure 2. Synthesis of tetrafunctional, COF-precursor monomers. (a) Tetrakis(4-aminophenyl)methane, and (b) tetrakis(4-formylphenyl)methane.
Figure 3. Solvothermal synthesis of COF-300 from tetrakis(4-aminophenyl)methane and terephthaldehyde. Reagents and conditions: 24mg (0.18 mmol) terephthalaldehyde, 40mg (0.11 mmol) tetrakis(4-aminophenyl)methane, 2 mL dioxane, 0.4 mL 3M acetic acid, 120°C for 3 days (As synthesized), immersion in THF for 24 hr (THF exchange), vacuum dry (0.1 mtorr) for 12 hr at RT and 2 hr at 100°C (Activated).
Table 1. Reaction conditions used for COF-300 synthesis.
| Solvothermal
| Sc(III)-catalyzed
| |
| B
| C
| D
|
Tetra(4-aminophenyl)methane
| 40 mg
| 51 mg
| 51 mg
|
Terephthaldehyde
| 24 mg
| 36 mg
| 36 mg
|
Sc(OTf)3
| -
| 5 mg (5 mol%)
| 5 mg (5 mol%)
|
Dioxane
| 2 ml
| 3 ml
| 3 ml
|
3 M Acetic acid
| 0.4 ml
| 0.6 ml
| -
|
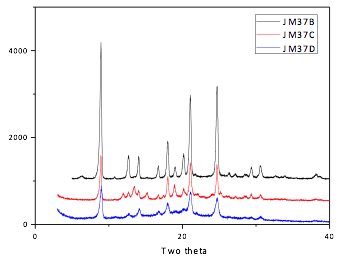
Powder x-ray diffraction (XRD) revealed that the materials obtained by the conventional solvothermal and Sc(III)-catalyzed syntheses exhibited very similar morphologies; however, the addition of acetic acid to the Sc(III)-catalyzed system resulted in extraneous peaks of unclear origin in the diffractogram (Figure 4). Comparison of the morphology and surface area of these materials with the CuAAC-generated MOPs is ongoing.
Figure 4. Powder x-ray diffractograms of COF-300 generated by (B) solvothermal synthetic conditions, and Sc(III)-catalyzed conditions in the (C) presence and (D) absence of 3 M acetic acid.