Reports: ND455820-ND4: Theoretical and Experimental Investigations of Carbocation Diradicals
Arthur H. Winter, PhD, Iowa State University
Carbocations are widely viewed to be closed-shell singlet electrophiles. Our computations supported by the PRF show that azulenyl-substituted carbocations have triplet ground states. This triplet ground state for azulenyl carbocations stands in striking contrast to the isomeric naphthenyl carbocation, which is a normal closed-shell singlet with a large singlet-triplet gap. Furthermore, substitution of the azulenyl carbocation can substantially alter the energy gap between the different electronic configurations and can manipulate the ground state towards either the singlet or the triplet state depending on the nature and location of the substituent. A detailed investigation into the origin of this spin state reversal, including NICS calculations, structural effects, substitution patterns, orbital analysis, and detailed linear free-energy relationships allowed us to distill a set of principles that caused these azulenyl carbocations to have such low-lying diradical states. The fundamental origin of this effect mostly centers on singlet state destabilization from increasing antiaromatic character, in combination with a smaller, but important, Baird triplet state aromatic stabilization. We find that azulene is not unique, as extension of these principles allowed us to generate simple rules to predict an entire class of analogous non-alternant carbocation and carbanion structures with low-energy or ground state diradical states, including a triplet cation with the highest known singlet triplet-gap for a hydrocarbon. Although these ions have innocuous-looking Lewis structures, these triplet diradical ions are likely to have substantially different reactivity and properties than typical closed-shell singlet ions.
The textbook view of the electronic structure of a carbocation is that of an approximately sp2 hybridized carbon with an empty p orbital. This simplistic picture stands in sharp contrast to the related nitrenes, carbenes, nitrenium ions, oxenium ions and other reactive intermediates that possess one or more lone pairs of electrons that can be distributed between two orbitals, allowing (at least) two closed-shell singlet configurations, an open-shell singlet configuration, and a triplet configuration, each of which potentially offers up a unique landscape of reactivity and properties. Since carbocations do not possess a lone pair, and given early theoretical investigations by Schleyer suggested that simple carbocations had large energy gaps to open-shell states, the simple view of the electrophilic closed-shell singlet carbocation has largely remained unchallenged.
Here, we demonstrate that carbocations conjugated to the non-alternant hydrocarbon azulene can have low-energy or ground state triplets depending on the point of attachment. A detailed investigation into the origin of this spin state reversal, including NICS calculations, structural effects, substitution patterns, detailed linear free-energy relationships, and orbital analyses, allowed us to distill a set of principles that explains why these azulenyl carbocations have low-lying diradical states. These principles allowed for the prediction of a set of analogous structures containing not only carbocations, but also carbanions that exhibit low-lying or ground state triplets using simple canonical structure ideas. These examples point to not only a new and potentially broad class of carbocations that defy the textbook paradigm that carbocations are electrophilic species with closed-shell singlet ground states, but also to a new class of carbanions that may have application to high-spin materials.
The linear-free energy relationships for various substituted azulenyl-cations are shown below:
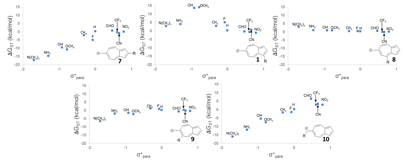 Linear free-energy relationships
of structures (UB3LYP/6-31+G(d,p)).
Linear free-energy relationships
of structures (UB3LYP/6-31+G(d,p)).
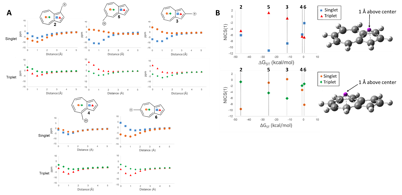
Understanding the origin of this remarkable spin inversion was performed by investigating the antiaromaticity using Nucleus Independent Chemical Shift calculations, shown in the figure below.
NICS values of the center of the 5-membered and 7-membered rings for structures 2-6 on the singlet and triplet surfaces. A) NICS value versus distance above plane of ring. B) NICS(1) value of 2-6 plotted against the ΔEST. (■ 5-Membered Ring Singlet, ▲ 5-Membered Ring Triplet, ● 7-Membered Ring Singlet, ♦ 7-Membered Ring Triplet) GIAO-HF/6-31+G(d,p)//CBSB7
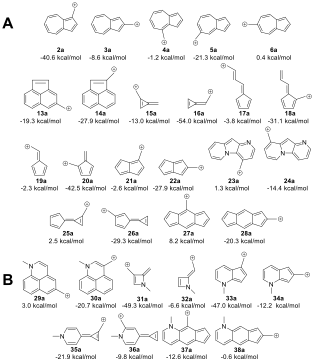
From these calculations we were able to predict a broad class of ions having low-energy diradical states. Some of these are shown in the figure below:
A) ΔEST of additional ions. Ions with formally Huckel antiaromatic/Baird aromatic canonical structures have low-lying diradical states. B) Manipulation of the ΔEST through the introduction of a 3° amine leads to a change in the favored spin state. UB3LYP/6-31+G(d,p)