Reports: UR152993-UR1: Aromatic Donor-Acceptor Organocatalysis: Noncovalent Activation of Aryl Halides in Green Palladium Cross-Coupling Reactions
Joseph J. Reczek, PhD, Denison University
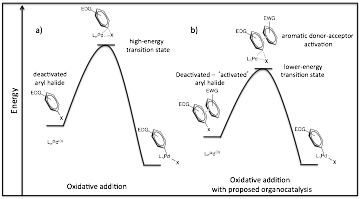
In the past year we have continued our work towards developing a general approach to the aromatic donor-acceptor organocatalysis of electron-rich (deactivate) aryl chloride coupling reactions. The overall plan is to lower the activation energy of the rate limiting oxidative addition step in these reactions by adding an electron-poor aromatic co-catalyst. In aqueous solvents, the complementary electrostatics of the aromatics will lead to face-centered stacking (solvophobic effect), possibly decreasing the C-Cl bond strength of the aryl chloride and driving the reaction (Figure 1).
Figure 1. Illustration of the first oxidative addition step in aryl halide cross-coupling reactions, highlighting the transition state energy for: a) a standard aryl halide palladium coupling, b) the proposed ADA-activated aryl halide coupling.
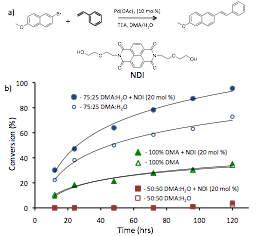
In initial studies, we observed a modest rate enhancement of a Heck coupling reaction in the presence of an electron-poor naphthalene diimide (NDI) co-catalyst (Figure 2). While promising, reaction times in this system were not conducive to timely study of conditions. Additionally, the poor water miscibility of the reagents (no reaction observed at 50% water) limited the solvophobic driving force for potential ADA catalysis that could be leveraged for this system.
Figure 2. a) Scheme for Heck reaction with NDI co-catalyst. b) Graph of GC-MS data following % conversion of six Heck reactions with and without the co-catalyst.
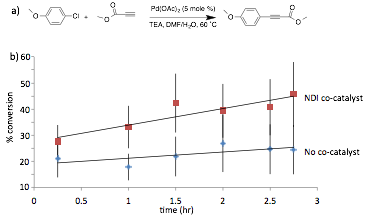
We next moved to exploring ADA catalysis in Sonogashira coupling reactions, which generally have significantly shorter reaction times compared to the similar-type Heck reactions. In the presence of the same NDI co-catalyst, we found conditions in which a rate enhancement trend was observed for the Sonogashira coupling in a reasonable time frame (Figure 3). However, solubility is still limited in this system, and the % conversion measured showed a high degree of variability, leading us to believe that we were not collecting homogeneous reaction samples.
Figure 3. a) Sonogashira coupling reaction initially explored. b) Percent conversation with and without NDI co-catalyst. The data sets shown are the average of 5 independent reactions under the same conditions. While promising, error bars indicate the high level of variation in our system, and limit the statistical significance of the observed rate enhancement.
We then turned to the synthesis of model reagents with high solubility in water in order to maximize solvophobic interactions and hopfuly increase the consistency of our reactions set up and analysis. While these reagents were soluble in water, we unfortunately found that the Pd(OAc)2 catalyst with TEA base was not compatible with these reaction conditions, giving highly inconsistent results and often little to no coupling product.
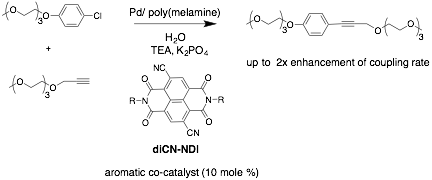
At the beginning of this reporting period we were successful in developing a catalyst system that consistently gave product for our water soluble model reagents in 100% water (Scheme 1). The Pd catalyst is made by first generating a poly-melamine scaffold to which Pd is complexed. The result is a polymer-supported catalyst that is completely water soluble and able to consistently give good yields in our Sonogashira couplings. We have tested this system with a number of co-catalysts, with especially promising results reaction seen with the diCN-NDI aromatic co-catalyst shown below. In one instance, we observed a 2x rate enhancement compared to the no co-catalyst control reaction with this system. Unfortunately, we are still plagued by inconsistencies in the initial rates of our coupling reactions, making it extremely difficult to collect data that tells a statistically significant story. We currently think this may be due to kinetic factors involved in some 'initiation' of our Pd catalyst of the coupling, leading to some rate variations inherent to the reaction. We are currently working on ideas to modify or circumvent this issue.
Scheme 1.
Impact:
In addition to the progress discussed above, this PRF funding has had a significant impact on Denison's department of Chemistry and Biochemistry. This grant has funded a student, Michelle Hill, in full-time undergraduate research for the summer of 2015, and for a 6-week period in the summer of 2016; she presented this work at the fall ABRCMS meeting. Michelle, now a senior at Denison, is an African American woman who is currently applying to Chemistry PhD programs. This funding has also contributed to chemicals and supplies for Michelle and two other undergraduate students participating in semester-research for the reporting period. This significantly enhances the research culture of the department at Denison, raises the visibility of the chemical sciences among students, and enhances the likelihood of students continuing in the sciences. On behalf of my students, and myself, I am thankful to PRF for the invaluable support of undergraduate research.