Reports: ND454262-ND4: Reaction Mechanism of C-H Activation of Fe-Catalysts
Junrong Zheng, Rice University
In the second fiscal year (09/2015-08/2016) supported by the ACS PRF grant PRF# 54262-ND4, we continued research on the mechanistic studies of formic acid decomposition by C-H activation. In previous year, we demonstrated that the 3D structure of a reaction intermediate which is too fast for NMR to measure in the reaction can be determined by our vibrational cross angle method. In this year, we investigated further in this direction.
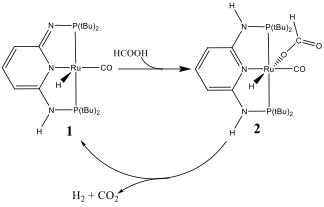
The reaction system investigated in this work is the generation of hydrogen gas by decomposing formic acid, catalyzed by a ruthenium PN3-pincer complex (1 in scheme 1). Formic acid, a major product of biomass processing and a formal adduct of H2 and CO2, has recently attracted considerable research interest. It is considered a potentially renewable liquid supply and storage material for hydrogen. A sustainable cycle can be envisioned using formic acid to supply hydrogen. To store hydrogen, hydrogen and CO2 are added together to form formic acid. To release hydrogen, formic acid is decomposed into hydrogen and CO2. The hydrogen storage density of formic acid is relatively high, 53g H2/L, suitable for automobile and portable applications. Therefore, even before formic acid can be produced from renewable sources with a reasonable cost, it is already very attractive to use formic acid as hydrogen source for many applications.
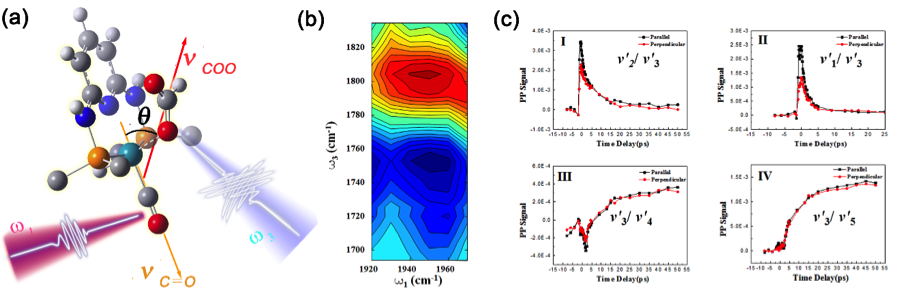
The decomposition of formic acid with the presence of catalyst 1 is illustrated in scheme 1 . When formic acid is dropped into a DMSO or DMF solution of catalyst 1 with the presence of weak base, hydrogen immediately liberates from the solution. 2 was proposed to be the key intermediate structure for the reaction. We have determined the 3D structure of 2 with our unique 2D IR setup and vibrational cross angle method and ab initio calculations (fig.1). From the structure and further studies, we deduced that by adding a weak base with a right pkb value, the system can provide the right concentrations of formate and donatable proton to dramatically improve the reaction rate and lifetime. Following such a reasoning, by adding K2CO3 into the system, the turn over number of the reaction is >1,000,000. Last year we built the first self-sustainable formic acid hydrogen fuel cell model car (12W) in the world. This year, with the improved catalytic system, we built a 300W formic acid model car which a 10-year kid can sit on and drive! (fig.2) Manuscripts from this project are under preparation and submission now
This model system study allows us to explore all aspects of our method in determining reaction intermediate structures, and demonstrate the power of this new technique to address both fundamental and practical problems, which will be applied to the studies of other catalytic systems including the Fe-nonheme C-H activation catalysts in the near future.
Scheme 1. The structures of the N3-pincer catalyst (1) and the major intermediate (2) of the decomposition of formic acid catalyzed by 1.
Figure 1. Cross peak measurements for vibrational mode pairs. (a) Illustrations of the cross angle vibrational coupling measurement method for vibrational modes carbonyl stretch (v2′) and C=O stretch (v3′). The t-Butyl groups have been omitted for clarity. (b) The 2D spectrum of the cross-peak for v2′ and v3′ at a waiting time 0.1 ps. (c) Plots of the polarization dependent cross peak intensities for four vibrational pairs with the polarization of the excitation beam parallel (//) and perpendicular (^) to the polarization of the detection beam, respectively.
Figure 2. 300W formic acid fuel cell car. The drive was Prof. Huang's son. The picture was taken at Rice University.