Reports: ND653264-ND6: Thermodynamic Insights into Surfactant Assembly in Protic Ionic Liquids
Henry Ashbaugh, PhD, Tulane University
The overarching goal of this proposal is to investigate the assembly of surfactants in protic ionic liquids and their mixtures with water to gain insight into the similarities and differences between micellization in aqueous and non-aqueous environments and potentially expand the utility of ionic liquids in petroleum product processing.
In year 1, I recruited a graduate student to work on the project. In that year she worked to develop and verify the molecular simulation force field to be able to simulate the properties of alkylammonium nitrate (AAN) based ionic liquids. The quality of the force field was judged based on its ability to reproduce the experimental mixture properties of ethyl- propyl- and butylammonum nitrate (EAN, PAN, and BAN, respectively) with water.
In year 2, we worked to study the properties of EAN, PAN, and BAN mixtures with water at solvating nonpolar gases to demonstrate both the poor solubility of nonpolar species in AANs as well as the characteristic thermodynamic properties, which are thought to mirror those of nonpolar solutes in water.
In year 3, we examined the structure of nonionic surfactant micelles in both EAN and water to determine the differences between these solvents as self-assembly media. To begin we place 40 C12E4 nonionic surfactants in close proximity to one another in a simulation box of EAN to examine the formation of a micelle. Our initial efforts were stymied by the fact that at room temperature the diffusion rate of the surfactants in the ionic liquid was so slow that the surfactants barely moved of the course of the simulation to form a simple micellar structure. This difficult was eventually overcome by establishing a heating and cooling protocol to form the micelle. No such procedure was required in water, as the solvent viscosity is low enough to permit rapid surfactant diffusion. Production simulations of micelles in both EAN and water were subsequently performed at a series of temperatures to extract thermodynamic results.
Given that the micelles in EAN and water were established to have the same aggregation number (40), the structural properties of the micelles were quite similar. The micelles were found to be approximately 20 in radius and effectively spherical in shape. As noted above, the higher viscosity of EAN minimized fluctuations in the micelle structural properties compared to water. Nevertheless, the average structural properties were quite similar.
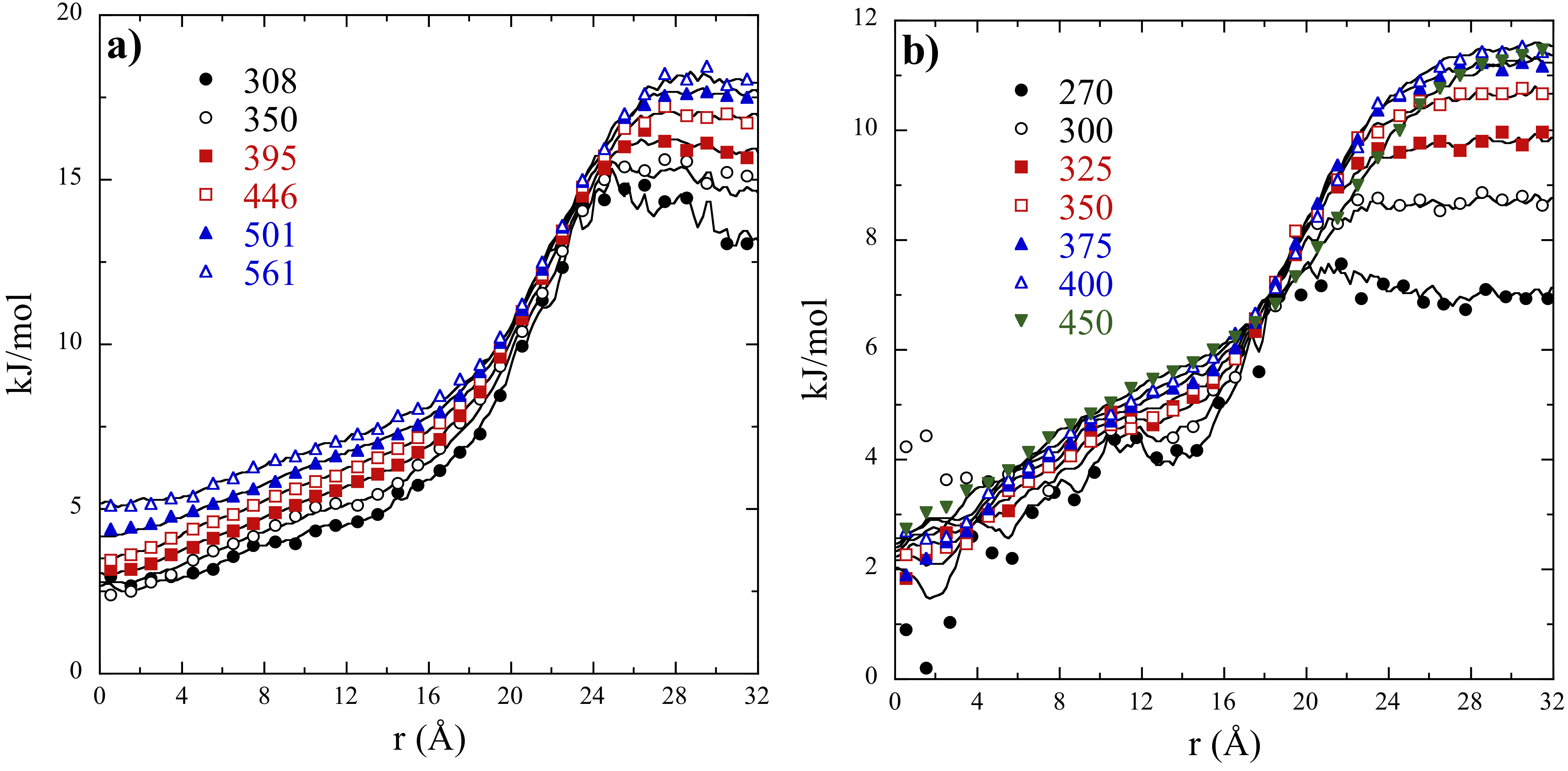
One of the practical uses of micelles in solution is as an emulsification agent, suspending nonpolar species in solution within the micellar cores. In Figure 1, we show the excess free energy of argon as a function of distance from the center of mass of micelle in EAN (Fig 1a) and water (Fig 1b) over a series of temperatures as determined from statistical thermodynamic particle insertion techniques.
Figure 1. Excess chemical potential as a function of distance from the center-of-mass of a 40-mer C12E4 nonionic surfactant micelle in EAN (a) and water (b). The simulations were conducted over a series of temperatures indicated in K in the figure legends. The points indicate our simulation results, while the lines indicate fits of eq. [1] to the temperature dependent simulation results in order to extract solvation enthalpies and entropies.
The results of these calculations show that argon in strongly attracted into the micelle interiors, as indicated by the lower free energies (chemical potentials) within the micelle interior for r < 20 . The extent of the attraction is strongest in EAN as a result of the considerably higher free energy of argon in bulk solvent (r > 24 ) compared to that in water. This reflects poorer solubility of argon in EAN from simulation compared to water.
The interaction free energies, G(r), are correlated by fitting
G(r) = A(r) + B(r)(T-T0) + C(r)Tln(T/T0) [1]
where A(r), B(r), and C(r) are fitted distance dependent functions, and T0 = 300 K is the reference temperature. Eq. [1] is obtained by assuming the heat capacity of interaction is independent of temperature. This equation was successfully used in year 2 to correlate the solubilities of methane and argon in AAN/water mixtures. The utility of this expression can be observed by its fidelity of reproducing the simulation results in Figure 1.
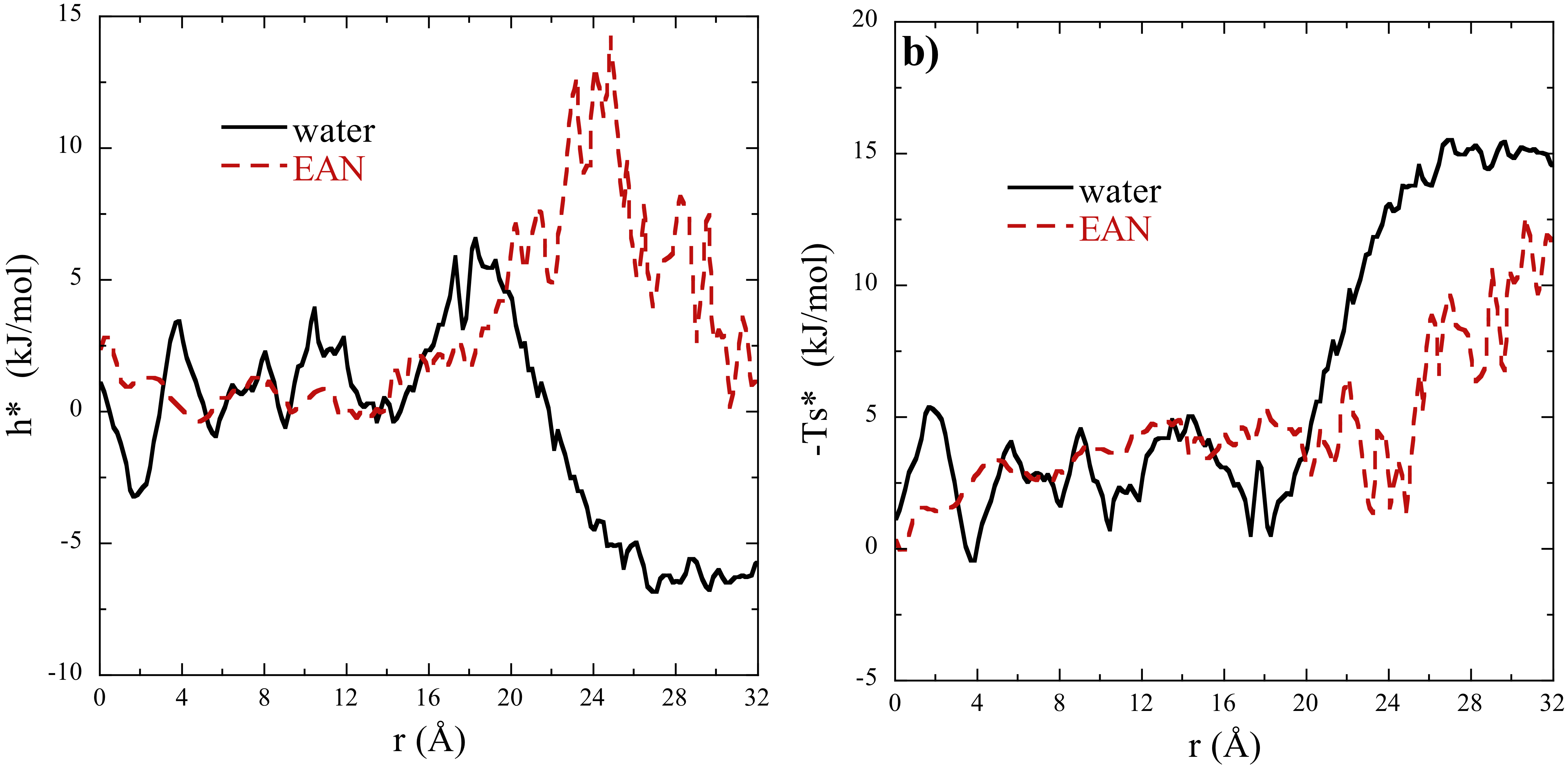
Using the fits of eq. [1] to our results we were able to extract interaction enthalpies (h=G + Ts) and entropies (s = -dG/dT) by taking the appropriate thermodynamic derivatives. In Figure 2 we report compare the interaction enthalpy and entropy for argon interacting with micelles in EAN and water.
Figure 2. Interaction enthalpy (a) and entropy (b) for argon interacting with micelles in water and EAN at 300 K. These thermodynamic properties were obtained from the temperature derivate of fitted function eq. [1]. The results for EAN and water are identified in the figure legends.
While the results in Figure 1 look similar for EAN and water, the enthalpies and entropies of interaction display distinct differences. For instance, while the solvation of argon within the micellar interiors in both EAN and water are close to zero, the enthalpy becomes large and repulsive out towards the bulk solvent in the case of EAN and large and attractive in the bulk solvent in the case of water (Figure 2a). Moreover, the interaction entropy is relatively small within the micelle interior in both solvents (Figure 2b). As argon enters the bulk solvent is it is entropically repelled by both solvents, although the effect is dominant in the case of water and smaller in EAN. Taken together, these results indicate that the thermodynamic properties of argon dissolution are less temperature dependent in EAN compared to water. We might expect a more robust temperature stability of dissolution in micelles in EAN. Interestingly the solvents have little impact on the solvation properties of argon within the micelles interiors, indicating that the micelles do an excellent job of shielding their interiors from the bulk solvent environment even in these distinct solvents.
This work were presented at the AIChE Annual Meeting 2015.