Reports: ND655242-ND6: Developing a Metal-Ion Burst Technique to Explore Ionic Strength Jump Dynamics
Nancy E. Levinger, Colorado State University
Debbie Crans, Colorado State University
This grant is developing a metal-ion burst experiment using ultrashort laser pulses to photolyze caged metal-organic complexes thereby releasing a burst of metal ions at a well-defined time. We will use the metal-burst to measure how metal cations form coordination complexes on very short timescales.
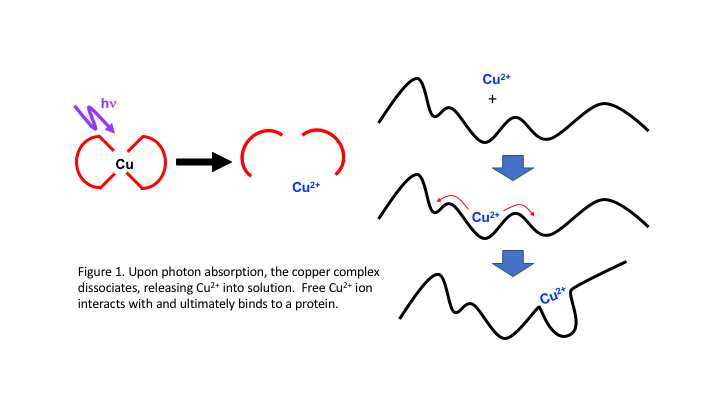
Much like photoinduced pH jump methods,1 we use short laser pulses to photoexcite molecules that dissociate, releasing metal cations that can subsequently bind to a target molecule. Delivered by an ultrashort laser pulse, we will measure binding events on timescales much shorter than currently possible. Ultimately, we plan to measure cation binding to proteins and gauge whether cation binds directly to the active site or binds first to other locations and subsequently moves to a stable configuration. Figure 1 shows schemes demonstrating these processes.
Specific aims:
1) Develop the metal-ion burst method initiated by photolysis of caged metal complexes to produce free metal cations in solution.
2) Characterize metal-ion burst through metal ion binding to sensor ligands in solution and in confined reverse micellar environments.
3) Apply metal-ion burst method to observe metal ion induced molecular aggregation in pre-aggregated states, bulk solution, and confined environments.
So far, we have focused on Aims 1 and 2.
Research progress:
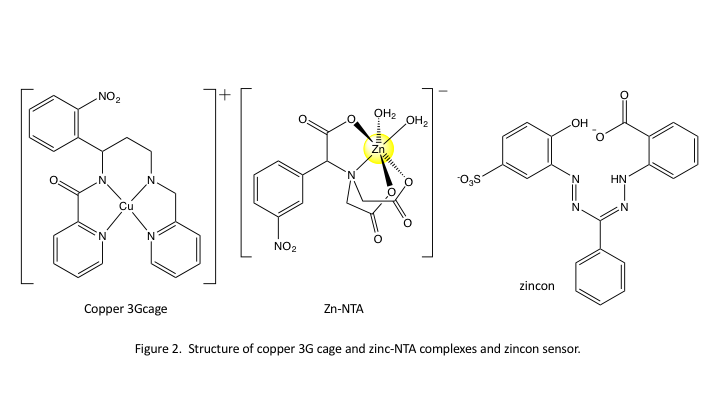
Initial experiments have utilized the copper3G cage molecule designed and synthesized by the Franz group2 and zinc-NTA complexes developed by the Burdette group3 (structures shown in Fig. 2) We performed ultrafast (10 fs-1 ns) and fast (>50 ns) transient absorption spectroscopy experiments to measure short time dynamics for release of Cu2+ and Zn2+ from the Cu3Gcage and Zn-NTA complexes. We photolyzed the complexes in bulk aqueous solution and in reverse micelles (RMs) in the presence and absence of a zincon 'sensor' ligand4 (structure shown in Fig. 2). Performing photolysis in AOT (sodium bis-ethylhexylsulfosuccinate) RMs, we intended to minimize diffusion by placing the metal cage complex and the zincon in close proximity. Unfortunately, thus far we have only been able to measure dynamics occurring on timescales ~30 seconds.
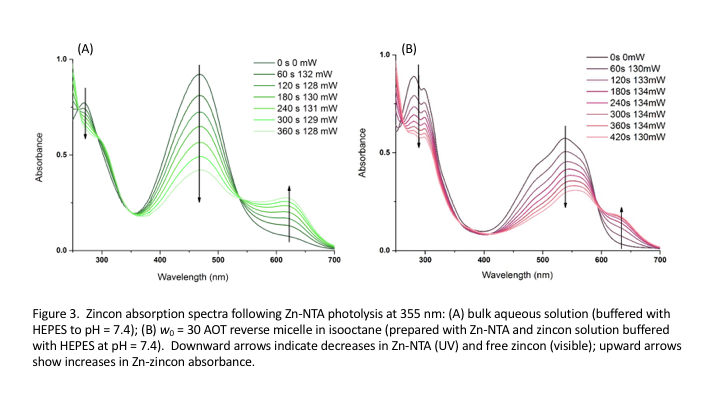
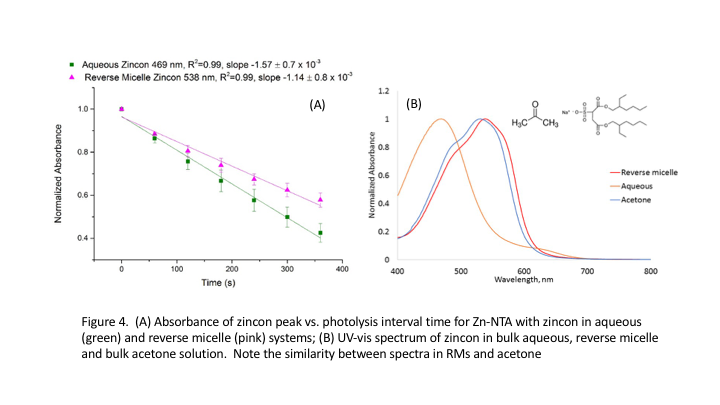
Although we have observed no evidence for ultrafast metal-ion bursts, we have observed interesting behavior of complexes, confirmed their ability to deliver metal cations both in bulk aqueous solution and confined in RMs, and demonstrated our ability to detect metal cations released into bulk aqueous solution and RMs. We have had most success with experiments releasing Zn2+ from Zn-NTA complexes and detecting released Zn2+ with zincon. We have demonstrated release of Zn2+ from the Zn-NTA complex in aqueous solution (buffered) and in RMs (AOT w0=30); following Zn-NTA photolysis (l=355 nm, Opolette laser) zincon bind the free Zn2+. The series of UV-vis absorption spectra (Fig. 3) decrease in free zincon and increase of Zn-zincon both in aqueous buffer solution and in RMs. The zincon absorption spectrum in RMs differs from the spectrum in bulk solution, Fig. 3B, shifting to longer wavelength and displaying structure absent in the bulk aqueous spectrum. The decay of the free zincon peak follows the binding kinetics the released Zn2+ cation, Fig. 4, and is minimally slower for the RM compared to bulk aqueous solution. This opposes our hypothesis that RMs would enhance binding by placing the dissociating complex and sensor ligand in close proximity
We explored the origin of differences between bulk aqueous solution and the RMs using NMR spectroscopy and UV-vis absorption spectroscopy of zincon and Zn-zincon in organic solvents. 1D 1H NMR of free zincon and Zn-zincon in bulk aqueous solution and in RMs show significant differences in peak positions and spectral bandwidth suggesting that zincon may embed in the interface. UV-vis spectroscopy shows surprising similarity between zincon RMs and bulk acetone, (Fig. 4B).
Impact on student education:
The research reported here has served to train students with laboratory and critical thinking skills. Dr. Richard Cole, in the Levinger group has led time-resolved spectroscopy studies, training graduate student Cheryle Beuning and several undergraduate students. Ms. Beuning has worked closely with undergraduate students who presented 3 posters at the CSU Celebrate Undergraduate Research and Creativity poster session, spring 2017. Ms. Beuning presented results at the 2017 spring national ACS meeting.5 Two undergraduate students will co-author the manuscript Ms. Beuning is currently writing about project results.
References
1. (a) Abbruzzetti, S., et al., 2006. Acid-induced unfolding of myoglobin triggered by a laser pH jump method, Photochem. Photobiol. Sci. 5, 621-628; (b) Donten, M. L., et al., 2011. A Consistent Picture of the Proton Release Mechanism of oNBA in Water by Ultrafast Spectroscopy and Ab Initio Molecular Dynamics, J. Phys. Chem. B 115, 1075-1083; (c) Gutman, M., et al., 1983. pH jump - kinetic-analysis and determination of the diffusion-controlled rate constants, J. Am. Chem. Soc. 105, 2210-2216.
2. Ciesienski, K. L.; Franz, K. J., 2011. Keys for Unlocking Photolabile Metal-Containing Cages, Angew. Chem.-Int. Edit. 50, 814-824.
3. Basa, P. N., et al., 2015. A Zinc(II) Photocage Based on a Decarboxylation Metal Ion Release Mechanism for Investigating Homeostasis and Biological Signaling, Angew. Chem.-Int. Edit. 54, 13027-13031.
4. (a) Platte, J. A.; Marcy, V. M., 1959. Photometric determination of zinc with zincon - application to water containing heavy metals, Anal. Chem. 31, 1226-1228; (b) Sabel, C. E., et al., 2010. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon, Anal. Biochem. 397, 218-226.
5. Beuning, C., et al., 2017. Photolysis of a Zn(II)-nitriliotriacetate cage in AOT/isooctane reverse micelles, Abstr. Pap. Am. Chem. Soc. 253, 1