Reports: DNI654437-DNI6: Fundamental Interactions Between Petroleum Ions and Gases
Matthew F. Bush, University of Washington
Petroleum is a complex mixture of molecules containing primarily carbon, hydrogen, nitrogen, oxygen, and sulfur. Characterizing this mixture is critical for processing petroleum into higher-value fuels and materials. The objective of this research is to use ambient temperature, low-pressure ion mobility mass spectrometry measurements in different gases and computational chemistry to understand the fundamental interactions between gases and petroleum ions. This fundamental investigation will (1) elucidate the optimal gas for separating petroleum ions, (2) reveal the extent to which independent measurements in different gases provide additional specificity for petroleum ion identification, and (3) more generally provide a framework for designing experiments for and interpreting the results from future investigations of petroleum samples using ion mobility mass spectrometry.
Technical Progress. Over two years of funding, we made significant progress in characterizing the effects of different drift gases (He, Ar, N2, CO2, and N2O) on the absolute collision cross sections of two classes of ions: the twenty common amino acids and a series of analogues of quinoline. We then developed figures of merit and a framework for characterizing the performance and selectivity of ion mobility separations in different gases. Finally, we evaluated existing theoretical tools for calculating collision cross sections.
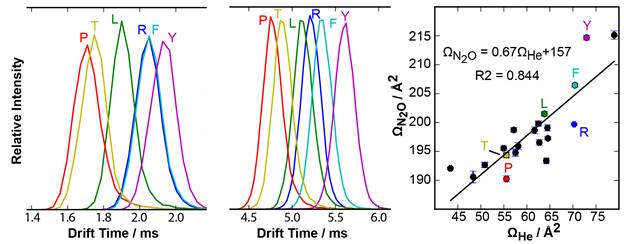
The twenty common amino acids were selected to probe the effects of a wide range of functional groups containing the same elements as petroleum. For each amino acid, the Ω increased with increasing polarizability of the gas. The Ω for all 20 amino acids in two different gases are correlated, but significant non-correlated differences were also observed. These non-correlated difference correspond to different specificities in different gases. For example, phenylalanine (F) and arginine (R) have indistinguishable drift times in He, but are separated in N2O. These differences are attributed to interactions that the ion has with He (which has a polarizability of 0.2 Å3 and no dipole moment) relative to those that it has with N2O (which has a polarizability of 3.0 Å3 and a dipole moment of 0.16 Debye). Using these results, we found that the peak capacity of each separation depended on factors contributing to widths of peaks as well as the range of Ω relative to the average Ω for the analytes. These factors favor N2 and Ar relative to the other gases. The number of pairs of amino acids that were resolved depended strongly on the peak capacity, but the identities of the pairs resolved also depended on the drift gas. Therefore, results using different drift gases are partially orthogonal and provide complementary chemical information.
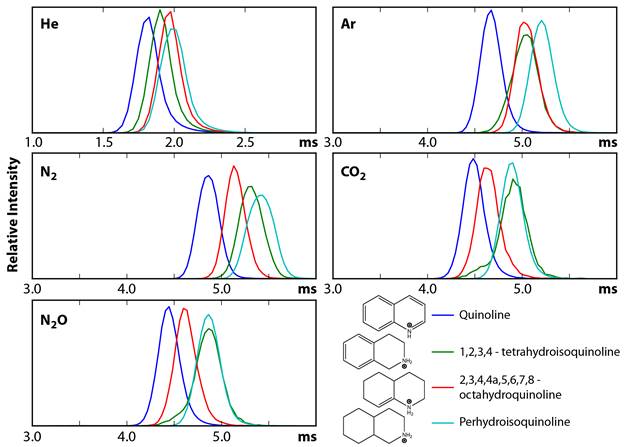
The analogues of quinoline were selected to probe the effects of gas polarizability on heterocyclic compounds that differ by the number of double bond equivalents (DBE), which is a critical classifier in petroleum analysis. Quinoline, 1,2,3,4-tetrahydroisoquinoline, 2,3,4,4a,5,6,7,8-octahydroquinoline, and perhydroisoquinoline have 7, 5, 3, and 2 DBE, respectively. The relative drift times of the protonated forms of these molecules depend on the drift gas used. For example, tetrahydroisoquinoline ions have shorter drift times than octahydroquinoline ions in He gas, but the opposite is true in N2. Clearly, differences in long-range, ion/molecule interactions can have a very significant effect on the mobilities of these ions. For these ions, existing theoretical approaches are adequate for predicting collision cross sections with He gas, but are inadequate for predicting those with the other gases.
We are expanding these studies to include additional analytes that will enable us to probe additional aspects of petroleum ion structure. Using those experimental results and complementary computational approaches, we will characterize the specific long-range ion/molecule interactions that give rise to drift-gas specific effects in ion mobility spectrometry.
Impact of Research. The collision cross sections measured here can be used to calibrate other low-pressure, ambient-temperature ion mobility experiments, including those using traveling-wave and trapped ion mobility spectrometry. Furthermore, these results provide invaluable benchmarks for new theoretical approaches for calculating the collision cross sections of ions in a wide range of buffer gases. Long term, these results will increase the information content available from analyses of petroleum samples using ion mobility mass spectrometry experiments.
Impact on Career of PI. This research has significantly expanded two areas of investigation for the PI: ion mobility mass spectrometry of small molecular ions and the effects of drift gas selection on ion mobility separations. Based on the outcomes of this research, the PI is pursuing new directions for improving the selectivity of ion mobility separations, applying ion mobility mass spectrometry to new analytes, and improving theory for predicting ion-neutral collision cross sections.
Impact on Career of Students. This research is an excellent training experience for Kim Davidson, the graduate student who performed all of these experiments, and Anna Bakhtina, an undergraduate student who assisted with these experiments. Based on these results, Kim Davidson published one article in Anal. Chem. as first author, will submit two additional manuscripts to peer-reviewed journals as first author, and been selected to speak at both the Lake Arrowhead Ion Chemistry and American Society for Mass Spectrometry conferences. More generally, her experiences pursuing this research have positioned her well for the remainder of her Ph.D. and post-graduate career. For Anna Bakhtina, her experiences pursuing this research will result in two second-author publications and will prepare her well for pursuing a Ph.D. in engineering.