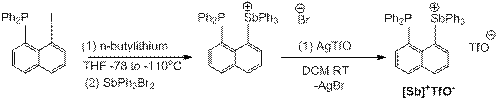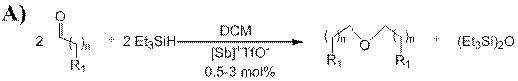Reports: UR354584-UR3: Antimony(V) Cations as Lewis Acid Catalysts for C-F Activation
Todd Hudnall, PhD, Texas State University
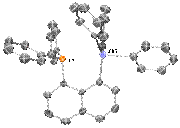
1.1 Overview of Research Efforts: During the first year of the funded proposal, we have worked on synthesizing two antimony(V) cations, complexes [Sb]+TfO- and [H-Sb]+TfO- (see Scheme 1). Both molecules have been fully characterized by HRMS and multinuclear NMR spectroscopy, and the former has been further characterized by XRD (Scheme 1, top right). These compounds are easily prepared by a step-wise lithium-halogen exchange from a lithiated naphthalene derivative and Ph3SbBr2, followed by halide abstraction with AgOTf. Our initial efforts were focused on developing FLP chemistry with [Sb]+TfO-. Our original hypothesis was that the neighboring Lewis basic phosphine and Lewis acidic antimony would result in a potent frustrated Lewis pair to enable the activation of robust small molecule. Unfortunately, these explorations have not been successful.
Scheme 1: Synthesis of 1-diphenylphosphinonaphthyl-8-triphenylstibonium triflate ([Sb]+TfO-) and 1- triphenylstiboniumnaphthyl triflate ([H-Sb]+TfO-).
1.2 Catalytic Applications of [Sb]+TfO- and [H-Sb]+TfO-: Depsite the lack of reactivity with small molecules, we found that both [Sb]+TfO- and [H-Sb]+TfO- serve as potent Lewis acid catalysts for a variety of transformations including the reductive coupling of aldehydes, self Aldol condensation and cyclcotrimerization of aldehydes (Scheme 2). These reactions were shown to take place at low catalyst loadings (0.5—3 mol%) and proceeded in good to excellent yields. Moreover, there is some selectivity observed between the two antimony cations, however these results are very new and we haven’t clearly determined what those specific differences are.
Scheme 2: A) selective reductive coupling, B) self Aldol condensation, and C) cyclotrimerization of aldehydes catalyzed by antimony(V) cations ([Sb]+)
{n = 2, 8, R1 = Me; n = 0, 2, R1 = Ph; n = 0; R1 = (Me)2CH, (Et)2CH, Cyclohexyl, (Ph)2CH, C6F5, (orto-Br)Ph, (m-Br)Ph, (m-F)Ph, (para-CF3)Ph, (para-NO2)Ph, and (para-Me)Ph} Yield > 90%
(R1 = Me, n =2, 8; R1 = Ph, n = 1, 2) Yield > 90% n = 2, 8, R1 = Me; n = 1, 2, R1 = Ph; n = 0, R1 = (Et2)CH, Cy, and (Ph)2CH}
Impressively, the cycloctrimerization reaction shown in Scheme 2C does not require the use of any solvent, and therefore we believe this to be a novel green synthesis of 1,3,5 trioxanes. In addition, the antimony(V) cations can catalyze the conversion of aldehydes to acetals in the presence of triethoxysilane (Scheme 3) without the need for any organic solvent.
Scheme 3: Solvent free conversion of aldehydes and (EtO)3SiH into acetals catalyzed by [Sb]+
1.3 Outcomes of Research: We prepared two manuscripts for publication, 1 was submitted and accepted for publication:
Ugarte, R. A.; Devarajan, D.; Mushinski, R. M. and Hudnall, T. W.* “Antimony(V) Cations for the Selective Catalytic Transformation of Aldehydes Into Symmetric Ethers, a,b-Unsaturated Aldehydes, and 1,3,5-Trioxanes” Dalton Trans. 2016, 45, 11150-11161.
And a second manuscript that is currently under review at Green Chemistry:
Ugarte, R. A.; Hudnall, T. W.* “Antimony(V) Catalyzed Acetalisation of Aldehydes: An Efficient, Solvent-Free, and Recyclable Green Process” Green Chem.. 2017, doi: GC-ART-12-2016-003629.