Reports: UR754033-UR7: Development of Supramolecular Polymers Based on Novel Methylene-Bridge-Linked Multi-Calixarenes
Jordan L. Fantini, PhD, Denison University
Work on the synthesis of new sulfonated dicalixarenes continued during the reporting period, and the titration data for establishing the binding properties of sulfonated dicalixarenes with with mono- and di-guest molecules in aqueous solution was reassessed.
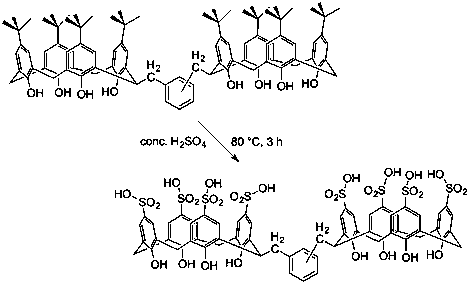
Following the synthesis of a fully sulfonated dicalixarene species which has two basket units oriented in opposite directions, and no linking group between them, we extended this to a series of dicalixarenes where calixarene units are joined by a xylyl group. The needed dicalixarenes were prepared by methods previously developed in our lab, and the sulfonations were initially carried out under conditions that were successful for synthesis of the fully sulfonated, directly linked dicalixarene species.
Figure 1. Intended synthesis of dicalixarene di-hosts with xylyl linkers (attempted for meta- and para-xylyl-linked species).
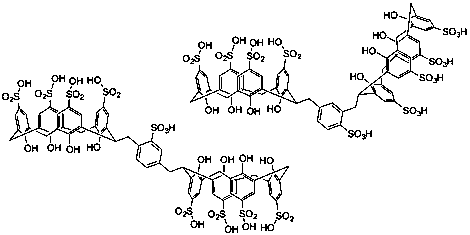
The conditions used for sulfonation of the baskets also proved sufficient to mono-sulfonate the central ring, thus products obtained were sulfonated as shown in Figure 2. Toward the end of the reporting period we found that calixarene sulfonation could be carried out under substantially milder conditions, namely concentrated sulfuric acid at ambient temperature. Future work will reveal whether these conditions spare the less activated linker ring from sulfonation.
Figure 2. Sulfonated dicalixarenes with sulfonated linkers.
The binding behavior of a directly linked sulfonated dicalixarene with the methylpyridinium cation and the methylviologen dication had been studied earlier in our lab but we had not correctly interpreted the 1H NMR titration data. Using the free fitting routines avavilable at http://app.supramolecular.org/bindfit/ we have found that the dicalixarene binds methylpyridinium and methylviologen in nonbuffered D2O with association constants of K1 = 9200 M–1; K2 = 1500 M–1 (methylpyridinium) and K1 = 8300 M–1; K2 = 4900 M–1 (methylviologen). These values should be considered preliminary until the studies are repeated.
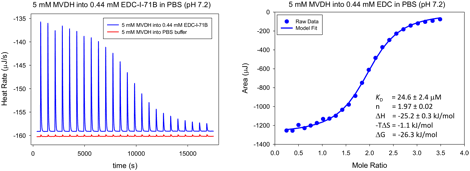
In collaboration with Dr. Kumar Sinniah (Calvin College), we have obtained isothermal calorimetry data for the association reaction of a dicalixarene and methylviologen in a pH 7.4 buffered solution. The experimental data are shown in Figure 3. Futher work with Dr. Sinniah will lead to insights on the thermodynamic behavior of other host–guest species, for example with the xylyl-linked calixarenes and also dimers of methylviologen and other di-guest species.
Figure 3. Isothermal titration calorimetry data for the association of the directly linked dicalixarene and methylviologen.
Further work will focus on obtaining more NMR and ITC titration data and syntheses of new dicalixarene host species.