Reports: ND1055049-ND10: Understanding a Novel Class of Highly Thermostable Liquids
Peter Khalifah, PhD, State University of New York at Stony Brook
In preliminary work prior to the start of this project, it was found that certain solid state reactions carried out within our group at elevated temperatures (≥ 600 °C) produced a liquid product rather than the expected polycrystalline powder product. The composition of this liquid product was unknown, though it was found to remain liquid over a very wide temperature range, including well below room temperature. The first objective in this project period was therefore to better understand the composition of this liquid.
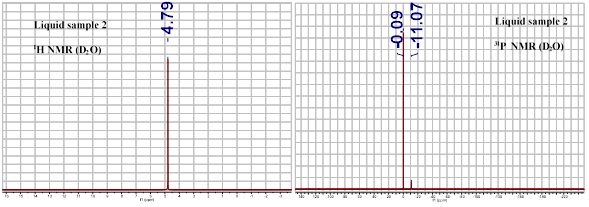
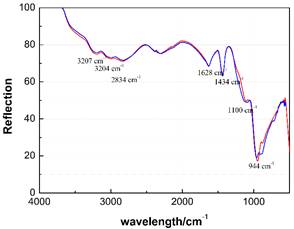
The clearest insights into the composition of this liquid came from 1H and 31P solution NMR (Figure 1). When the thermostable liquid was dissolved in D2O, one 1H and two 31P peaks were observed. The first 31P peak could be assigned to PO43- ions (present as phosphoric acid), while the second could not be matched to known species, but based on knowledge of the synthesis and chemical bonding principles was assigned to the nitridophosphate anion, PO3N4-, a complex oxoanion whose solution properties have never previously been reported. Investigations of this liquid through infrared spectroscopy (Figure 2) are also consistent with a mixture of PO43- and PO3N4- species. The spectrum of the liquid closely resembles that of a pure PO43- solution, but with a few slight shifts in peak intensity and position. The attribution of the thermostable liquid to a mixture of PO43- and PO3N4- species is consistent with the properties measured for this liquid, including a freezing point which is near but significantly lower than that for aqueous H3PO4 at any molarity, the rheological response of this liquid, and the very low pH of the thermostable liquid.
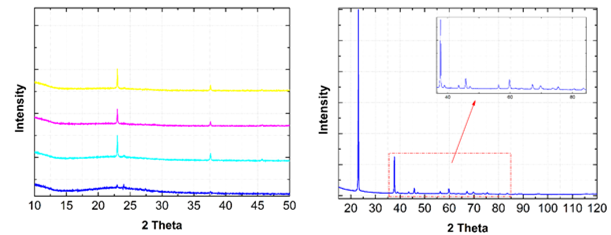
Since the PO3N4- species were present in low concentrations, synthesis efforts have been carried out to enhance the yield of this novel solution anion. The initial synthesis route could not be easily reproduced and tuned, so efforts were then refocused on the synthesis of the known oxynitride compound PON and its use as a precursor. Depending on the synthesis conditions, either crystalline or amorphous forms of PON could be produced (Figure 3). The crystalline phase was found to be well-indexed by a cristobalite-type structure with the tetragonal space group of I-42d (#122) and lattice parameters of a = b = 4.619 Å and c = 6.981 Å. The structure, chemistry, and chemical transformations PON are being further investigated.
Figure 1. 1H (left) and 31P (right) spectra of the thermostable liquid. The 31P peak at -0.09 ppm was assigned to PO43- ions, while the peak at -11.07 ppm was assigned to PO3N4-.
Figure 2. Comparison of the infrared spectra of a sample of PO43- (red) and a sample where both PO43- and PO3N4- species are present (blue).
Figure 3. Laboratory X-ray (Cu Ka) diffraction patterns of nominal “PON” samples synthesized under different conditions. Patterns either appeared crystalline (yellow, magenta, cyan scans) or amorphous (blue scan). The crystalline samples had a dominant peak just above 20° 2q, but long scans allowed a number of weaker peaks to be resolved and quantified.