Reports: ND755593-ND7: Mesoporous Polymer Frameworks by Architectural Control
Javid Rzayev, State University of New York at Buffalo
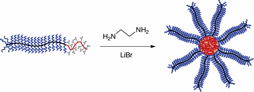
We have developed a new method for the formation of arm-first core-cross-linked star-brush polymers by using end-reactive bottlebrush copolymers precursors (Figure 1). Reactive bottlebrush-linear block copolymers with poly(methyl methacrylate) side chains and linear poly(glycidyl methacrylate) (PGMA) end blocks were utilized for the fabrication of brush-star architectures. Well-defined bottlebrush copolymers with reactive end blocks were prepared by a grafting-from method, where a block copolymer backbone was synthesized by sequential reversible addition-fragmentation chain transfer (RAFT) polymerization of functionalized monomers, while side chains were installed by atom transfer radical polymerization (ATRP) of methyl methacrylate initiated from the bromide groups of the backbone. Star formation was conducted in the presence of ethylenediamine as the cross-linker and LiBr as the catalyst at room temperature. While stoichiometric ratios of [EDA]:[oxirane] led to the best results, effective cross-linking of bottlebrush arms was observed even when a large excess of EDA was utilized, suggesting a possible polymer modification induced phase separation, which enhanced the star formation process. An interplay between the length of the reactive poly(GMA) end block and the length of bottlebrush side chains controlled the efficacy of star formation, which was explored by cross-linking a series of end-reactive bottlebrush precursors with varying structural parameters. TEM analysis of star-brush copolymers evidenced the formation of uniform spherical particles whose dimensions were consistent with fully stretched bottlebrush copolymer arms. The developed method provides means to connect giant bottlebrush copolymer arms (Mn > 300 kg/mol) into a star architecture in an effective and tunable manner. The results of these investigation were recently published (Altay, E.; Rzayev, J. Polymer 2016, 98, 487-494).
Figure 1. Formation of star-brush copolymers from end-reactive bottlebrushes.
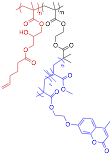
Due to slow cross-linking kinetics of the previous system, it was not suitable for the formation of bottlebrush copolymer networks. Therefore, we continued working on developing a more efficient and robust methods for bottlebrush end group cross-linking that can be utilized for network formation. For this purpose, we synthesized a new bottlebrush system that contained olefinic groups within a reactive end block and photo-cross-linkable coumarin groups as pendant functionalities on bottlebrush side chains. Oxirane groups of poly(GMA) blocks were reacted with pentenoic acid to introduce unsaturated double bond to the end-blocks for further intermolecular cross-linking via cross-metathesis. The coumarin moieties were incorporated into the side chains to take advantage of [2+2] cycloaddition induced by photo-crosslinking at 365 nm to achieve more rigid and stable linker between the chemically cross-linked cores expecting to decrease the amount of shrinkage during drying process. Diblock bottlebrush copolymers with reactive poly(GMA) end-blocks were initially taken as a model to optimize cross-linking conditions as the resulting star-brushes have good solubility in organic solvents and are easily amenable to characterization.
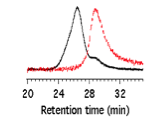
Cross-linking using cross-metathesis proved to be very effective. Star formation reactions proceeded at room temperature at very low polymer concentrations (~ 4 wt%). Rapid intramolecular cross metathesis of olefin groups in the end-blocks produced star-brushes in 5 hr with very high conversions (>90%) and low dispersities (Figure 2). The effectiveness of the reaction was not diminished by increasing the length of the backbone or side chains.
Figure 2. End-reactive bottlebrush copolymers with olefinic groups, and SEC characterization of their cross-linking in the presence of 2nd generation Grubbs' catalyst (red: before, black: after).
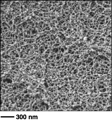
In order to extend this newly developed cross-linking chemistry for the formation of bottlebrush networks we synthesized bottlebrush copolymers with reactive linear blocks at both ends. A combination of controlled radical polymerization techniques was used to prepare the desired copolymers with olefinic reactive groups in the linear blocks and photo-cross-linkable coumarin groups in the middle bottlebrush block. Exposure of these copolymers in concentrated solutions to Grubbs' catalyst resulted in gelation. The reaction was allowed to proceed overnight at room temperature to ensure maximum conversion. Subsequent photoirradiation, solvent exchange and drying resulted in mesoporous monoliths with limited shrinkage (Figure 3). SEM analysis revealed the formation of a uniform, continuous network morphology with pore dimensions ranging from 10-50 nm. Nitrogen gas adsorption methods were used to characterize surface area of the fabricated mesoporous materials. For most monoliths prepared by variation of bottlebrush backbone and side chains BET surface areas were around 100 m2/g, while BJH pore sizes ranged from 9 to 35 nm, consistent with SEM results. These promising results demonstrate the feasibility of the preparation of controlled mesoporous polymer networks by rational design of bottlebrush copolymer building blocks.
Figure 3. Synthesis and SEM characterization of mesoporous polymer frameworks from end reactive bottlebrush copolymers.