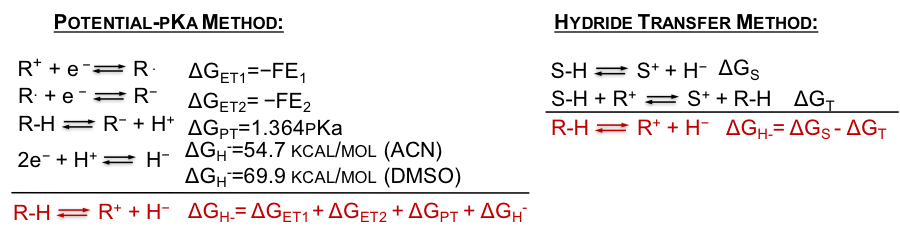Reports: ND454436-ND4: Concerted Excited-State Hydride Transfer from Organic Models
Ksenija D. Glusac, Bowling Green State University
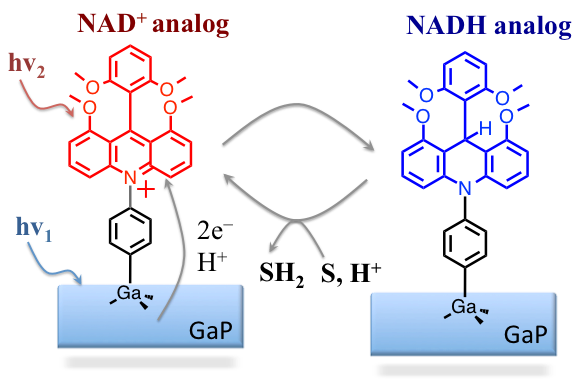
Introduction: The aim of this project is to study photocatalytic systems able of performing hydride-transfer reactions to appropriate substrate S, as presented in Figure 1. To develop the desired photocatalyst, three projects are currently underway in the Glusac labs: (i) Dye sensitization: The goal of this project is to find ideal conditions for efficient photoinduced electron transfer from excited NAD+ analog dye to the GaP; (ii) Two-for-one photoreduction: The current doubling mechanism (two-electron photosensitization) will be used to convert the NAD+ analog dye to the NADH analog; (iii) NADH analogs for proton reduction: The NADH-analogs will serve as reducing agents for the reduction of relevant substrates. In the previous report, we outlined the preliminary study of GaP sensitization using several NAD+ analogs. In this report, we describe the studies evaluating the hydricities of model NADH analogs.
Figure 1. A schematic representation of proposed photohydrides catalysts using NADH/NAD+ analogs.
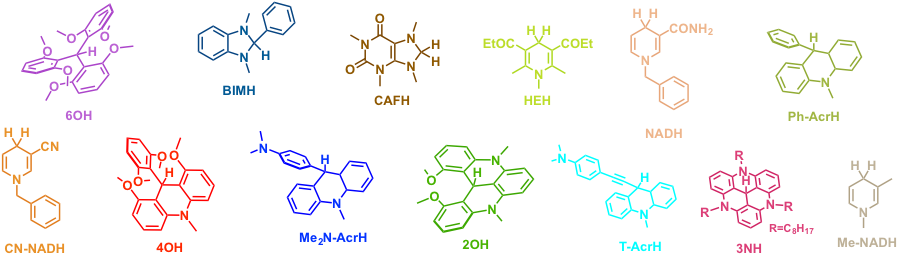
Research Summary: To evaluate the ability of model NADH analogs to reduce substrates, the hydricities of compounds presented in Figure 2 were evaluated using experimental and computational approaches. The hydride donating ability of a molecule is often described by its hydricity, which is defined as the Gibbs free energy for a hydride-ion release from the compound:
The lower values of hydricity indicate better hydride donors, and this thermodynamic parameter provides useful information on potential application of hydrides in reductions of appropriate substrates.
Figure 2. Structures of model NADH analogs investigated in this study.
The calculations of all thermodynamic parameters needed to obtain the hydricites were performed at the wB97x-D/6-311++G(2df,p)/CPCM level of theory, except for the solvation energy of hydride ion. The solvation energy for hydride ion in acetonitrile and dimethyl sulfoxide was derived using experimental values for one-electron reduction potential of hydrogen atom in the gas phase and solution.
Table 1. Calculated hydricities of model NADH analogs in acetonitrile and DMSO.
Compound | DGHyd (kcal/mol) | Compound | DGHyd (kcal/mol) | ||
DMSO | ACN | DMSO | ACN | ||
6OH | 84.1 | 85.8 | CN-NADH | 66.5 | 68.5 |
BIMH | 48.4 | 50.3 | 4OH | 73.2 | 75.1 |
CAFH | 51.3 | 53.2 | Me2N-AcrH | 70.3 | 72.2 |
HEH | 60.6 | 62.5 | 2OH | 61.1 | 62.9 |
NADH | 58.3 | 60.3 | T-AcrH | 64.7 | 66.6 |
Ph-AcrH | 72.8 | 74.9 | 3NH | 46.9 | 48.7 |
Me-NADH | 50.3 | 52.2 |
| ||
Several conclusions can be drawn from the calculated hydricities for our model NADH analogs (Table 1). First, the values are in the 47-86 kcal/mol range, which is comparable to the range reported for transition metal or boron based hydrides. For example, the hydricity of sodium borohydride, a well-known reducing agent, is 50 kcal/mol, which is similar to our CAFH or BIMH model compounds. Second, the molecular framework plays a role in hydricities: acridine-based models (such as Ph-AcrH) are generally weaker hydride donors than pyridine-based models (such as NADH), and these are in turn weaker donors than benzimidazole models (such as BIMH).
To evaluate the accuracy of hydricity values presented in Table 1, we obtained the experimental hydricities of the model compounds. The experimental hydricities were obtained using one of the two methods (Figure 3): (a) potential-pKa method, in which the hydricity is obtained from two one-electron reduction potentials of NAD+ analogs and the pKa value of the NADH analog; (b) hydride transfer method, in which the hydricity of a model compound is obtained by determining the equilibrium constant of a reaction with a hydride acceptor with known hydricity.
Figure 3. Methods for experimental determination of hydricites.
The experimental methods presented in Figure 3 were limited by the following: (i) the potential-pKa method was limited by the irreversibility of some of the reduction peaks or the fact that the second reduction peak (E2) of some model compounds was out of the electrochemical solvent window; (ii) the hydride transfer method was limited by the side chemical reactions that prevented the determination of the equilibrium constant. Despite these challenges, some of the experimental hydricites were obtained and were found to correlate reasonably well with the calculated values (Table 2).
Table 2. Experimental hydricities of model NADH analogs in acetonitrile and DMSO or ACN.
Compound | DGHyd (kcal/mol) | |
Exp | Cal | |
4OH/DMSO | 83.7 | 84.0 |
Me2N-AcrH/DMSO | 70.0 | 73.1 |
2OH/DMSO | 58.3 | 61.1 |
2OH/ACN | 60.0 | 62.9 |
NADH/ACN | 59.0 | 60.3 |
This preliminary study has identified the structural motifs needed for good hydride transfer reagents. We are currently in the process of determining the experimental hydricities of the remaining model compounds.
Future Work: The work presented above demonstrates that the model NADH analogs can be made with sufficient hydricities to reduce relevant substrates. The future studies will investigate the conditions for efficient two-electron, proton-coupled photoreduction of NAD+ analogs to form the appropriate NADH models. In addition, we will investigate the reduction of petroleum-related chemicals using our NADH analogs, with the goal of developing inexpensive earth-abundant catalysts for photochemical reductions of interest.