Reports: DNI554763-DNI5: Direct Structural Characterization of Individual Asphaltenes Using Scanning Probe Microscopy and Interface-Sensitive Techniques
Shelley Claridge, PhD, Purdue University
Scanning probe imaging of asphaltenes
In this project period, we have developed techniques for delivering, assembling, and controlling distribution of amphiphiles on layered materials. Because asphaltenes have amphiphilic structures, and can undergo significant π-stacking interactions, they are challenging to distribute on both hydrophilic and hydrophobic surfaces. Two techniques we have been developing within the context of other projects in the group have been applied to asphaltenes to change the molecular distribution on layered material surfaces, with the aim of reducing aggregation that inhibits structural characterization.
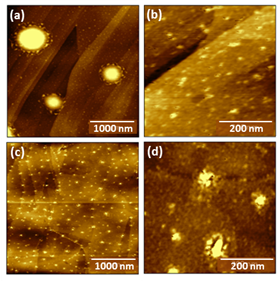
Figure 1. AFM images of Maya (Mexico) asphaltenes drop-cast from toluene onto HOPG at concentrations of: (a,b) 0.01 mg/mL, (c) 0.005 mg/mL, or (d) 0.001 mg/mL.
The two techniques we have investigated involve controlling the surface chemistry of the receiving surface, and controlling sample transfer conditions. Previously, we had observed that drop-casting samples of Maya (Mexico) asphaltenes acquired from ConocoPhillips through collaborator Hilkka Kenttamaa still distributed asphaltenes in large aggregates (typical diameters 300-500 nm, with heights of 10-30 nm) when deposited at concentrations at 0.01 mg/mL. Larger aggregates were interspersed with smaller aggregates with diameters > 20 nm, and heights > 5 nm. Reducing sample concentrations to 0.005 mg/mL and 0.001 mg/mL resulted in the two AFM images on the bottom row (left = 0.005 mg/mL, right = 0.001 mg/mL).
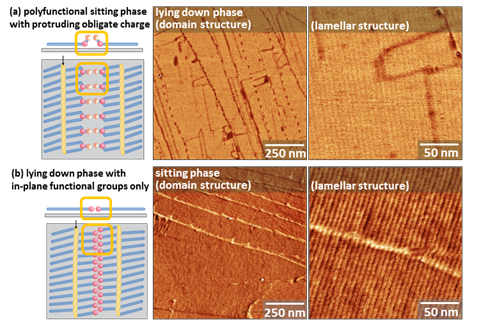
Reasoning that the molecules might distribute differently on an amphiphilic surface that displayed structured hydrophilic and hydrophobic regions at very small scales similar to those observed in asphaltenes, we screened samples of asphaltenes against two surface chemistries we have developed in our group for controlling interfacial wetting. Our group has worked extensively with lying down and sitting phases of polymerizable amphiphiles (Figure 2), and has discovered that they have different impacts on interfacial wetting depending on headgroup structure. Lying-down phases of carboxylic acids create a striped surface in which 1-nm wide hydrophilic stripes alternate with 5-nm wide hydrophobic stripes created by the long lying-down alkyl chains. Sitting phases are created when the amphiphile has a chiral, polyfunctional head, which must then protrude from the surface. Because this structural difference amplifies the chemical orthogonality between the hydrophilic head and hydrophobic tail, we have observed in other molecular systems that there can be substantial differences in solvent wetting and droplet spreading.
Figure 2. Lamellar structures of lying-down and sitting monolayers of amphiphiles.
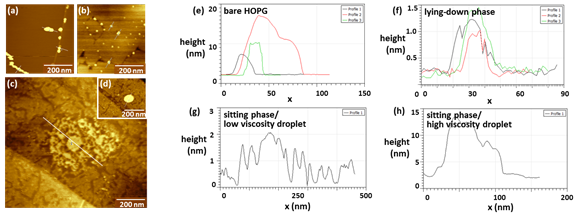
These experiments have important implications for controlling the aggregation or spreading of asphaltenes. In one set of experiments we have observed that spray deposition of asphaltene solutions at up to 0.5 mg/mL can result in pinning of droplets at step edges on HOPG (Figure 3, a,e), and primarily at domain edges for lying-down phases (Figure 3b,f). In contrast, on amphiphilic sitting phases, we observe spreading of asphaltenes to film thicknesses of ~1 nm for suitably low-viscosity droplets (Figure 3c,g), while higher-viscosity droplets adopt a geometry more similar to a spherical cap, with thicknesses of 10-30 nm (Figure 3d,h). The ruptured thin-film geometry observed under controlled spray deposition conditions (bottom image) represents a significant departure from the standard spherical cap morphology, suggesting more facile structural characterization.
Figure 3. Asphaltenes spray-coated onto: (a) bare HOPG, (b) lying-down phases, and (c,d) sitting phases of amphiphiles.
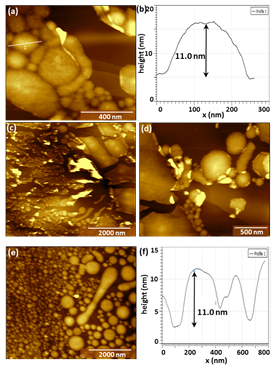
In this project period, we also examined the feasibility of spreading asphaltenes on layered material interfaces via Langmuir-Schaefer transfer using specialized sample transfer control methods developed in the lab that have been useful in increasing ordering in other types of amphiphilic molecules. While these methods produced changes in the transferred layer morphology in comparison with either drop-casting or standard L-S transfer protocols (Figure 4), the overall results from spray deposition appear more promising due to the reduction in film thickness.
Figure 4. AFM images and line scans associated with asphaltenes deposited on HOPG through Langmuir-Schaefer transfer.
In the no-cost extension period, we will examine differences in interfacial spreading of asphaltene droplets based on amphiphile chain length and asphaltene source, to optimize spreading behavior. Our hypothesis is that spreading will be facilitated under conditions in which there is structural similarity (conceptually analogous to epitaxial matching) between the striped amphiphile monolayer periodicity and the mean distance between polar/ionic groups in the asphaltenes. Asphaltene samples will also be characterized by high-resolution scanning probe microscopy to understand characteristic differences in visible structure based on asphaltene source.
Impact on the PI and supported student careers:
The PI is developing a research program that seeks both to understand and to control amphiphilic chemistries at interfaces. In the work funded through this project, the PIs group has begun a collaboration with prominent asphaltene researcher Hilkka Kenttamaa. In the past year, the PI’s work on interfacial patterning has gained support through an NSF-CAREER award, providing a long-term path for continuing studies of amphiphilic interface chemistry. The early impacts of the PI’s work have been recognized with the Purdue Early Career Award.
For the student supported in the past year, Jae Jin Bang, protected research time provided by the grant support has enabled her to make a number of key contributions. Her contributions to the understanding of the relationship between lying down and sitting phases have resulted in authorship of a manuscript published in Journal of the American Chemical Society.