Reports: ND555120-ND5: A Microrheological Study of Interfacial Nanoparticle Layers: Understanding Glassy Rheology, Aging and Stability
John C. Crocker, PhD, University of Pennsylvania
The goal of this project is to understand the interfacial rheology of dense collections of amphiphilic nanoparticles on oil water interfaces, informed by the hypothesis that they may resemble soft glasses, such as foams and slurries. This follows up on an earlier study done on a nice model system, small gold nanoparticles with grafted undecane-tetraethylene glycol ligands (i.e. in the CnEm family, specifically C11E4), undertaken with Dr. Kathleen Stebe. The earlier study probed Gibbs layers formed from these suspensions in a pendant drop apparatus, determining the equilibrium pressure-density isotherm and interfacial particle pair potential. The use of the pendant drop method, however, precluded the measurement of interfacial rheology. The current study seeks to probe this system using state of the art interfacial microrheology which will require small probes to provide the needed sensitivity. Towards this end, a new miniaturized liquid-liquid interface chamber suitable for microscopy must be produced, and a laser illuminated dark-field microscope must be assembled to reliably image the sub-micron probes. During the first funding period considerable progress was made on both instrumentation goals. This project is a key new research direction for the PI, which builds upon his earlier expertise in microrheology and microscopy. The postdoc on the project, Dr. Mehdi Molalei, has experience with holographic microscopy and particle tracking, is well-suited to this ambitious project, and aspires to future publications that advance the state of the art in this area.
A new instrument for high performance microscopy on controlled liquid interfaces
Many instruments have been developed to form and manipulate molecular or colloidal layers on liquid-air and liquid-liquid interfaces and to simultaneously measure the resulting interfacial tension, most notably the Langmuir trough. While the flat interface of such devices allows microscopy, it is common that vibrations, air currents and Marangoni stresses can cause drift and oscillatory motion, even after extraordinary care to limit external perturbations. As a result, the most high performance types of microscopy, such as the nanometer-scale tracking desirable for microrheology, can be very challenging. To advance this field, we designed and constructed a device that provides much of the functionality of a Langmuir trough, but which is only a few centimeters across, which greatly eliminates the troublesome oscillatory motions due to low-frequency vibrations and drifts and greatly reduces the amount of sample required. The wet portion of the instrument is produced using 3d printing, allowing inexpensive production of devices that can be simply discarded should they become fouled.
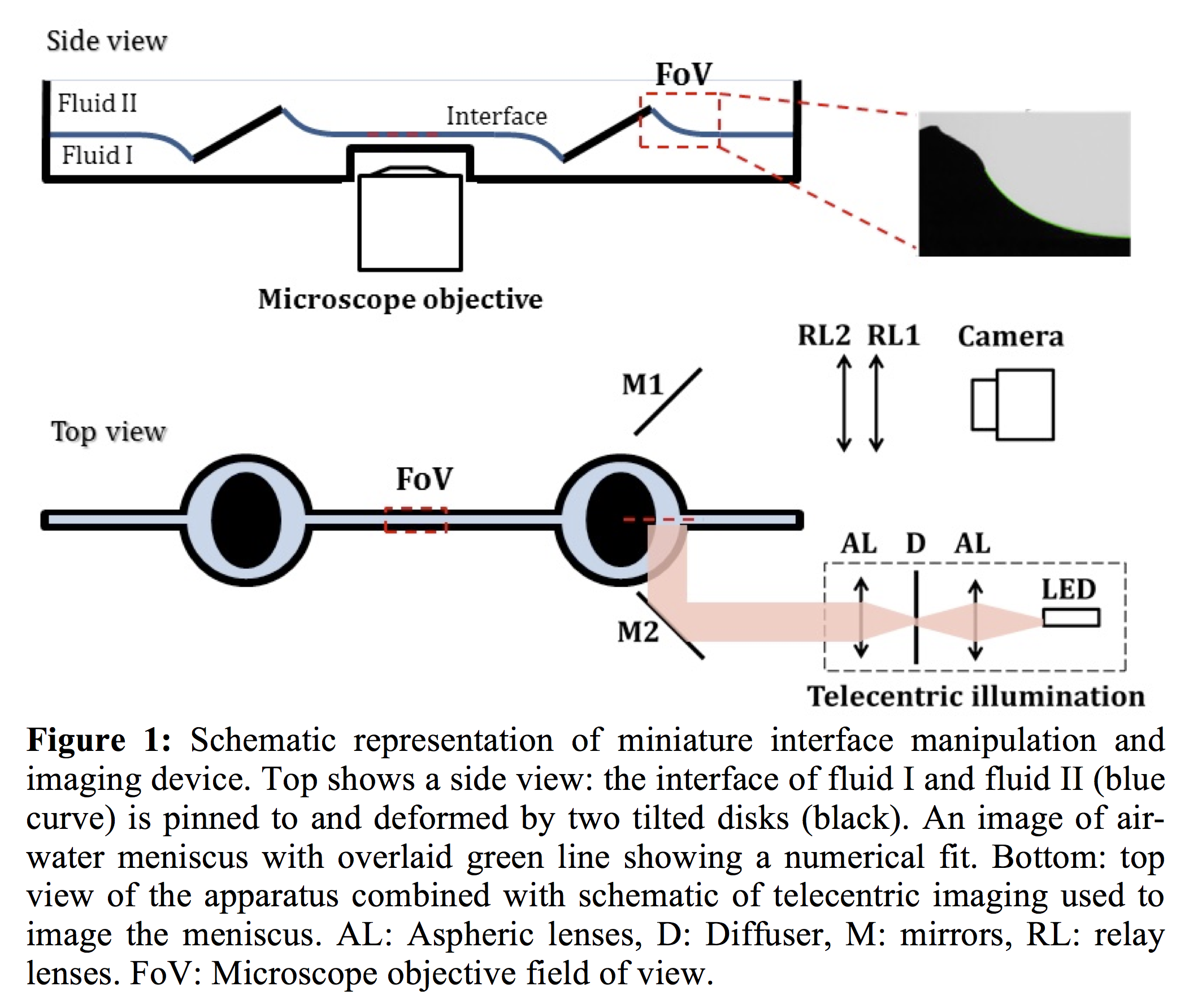
A schematic representation of the new apparatus is shown in Figure 1. The working concept of the setup is similar to the pendent drop apparatus since in both techniques the distortion of the interface under gravity is imaged to measure the interfacial tension. The competition between gravity and interfacial tension is characterized by a Bond number. We compare the shape of the meniscus to a numerical model based on Surface Evolver, to determine the experimental Bond number, from which the tension can be computed in real time. In practice the resulting precision is similar to that of the pendant drop apparatus.
Our device has several notable advantages. In the middle of the trough, the interface is flat which provides optical access to image the interface by high magnification, short working distance objectives. Tilting of the two disks pinned in the interface deforms the interface, allowing the interfacial area to be increased or decreased, as in a Langmuir trough. By using two disks on the either sides of the interface we eliminate flow from one side of trough to the other. As in a Langmuir trough, we can spread molecules or particles on the subphase to form a Langmuir monolayer. After forming the monolayer we can add another fluid on top of the interface. Working with particle laden fluid-fluid interfaces is technically challenging at best, and not possible with many simple Langmuir troughs. Lastly, due to its small size the new apparatus can be set up on various microscope stages. The trough being small minimizes sensitivity to vibration, reducing measurement error and facilitating precision tracking.
A custom Dark Field Microscope for interfacial particle imaging
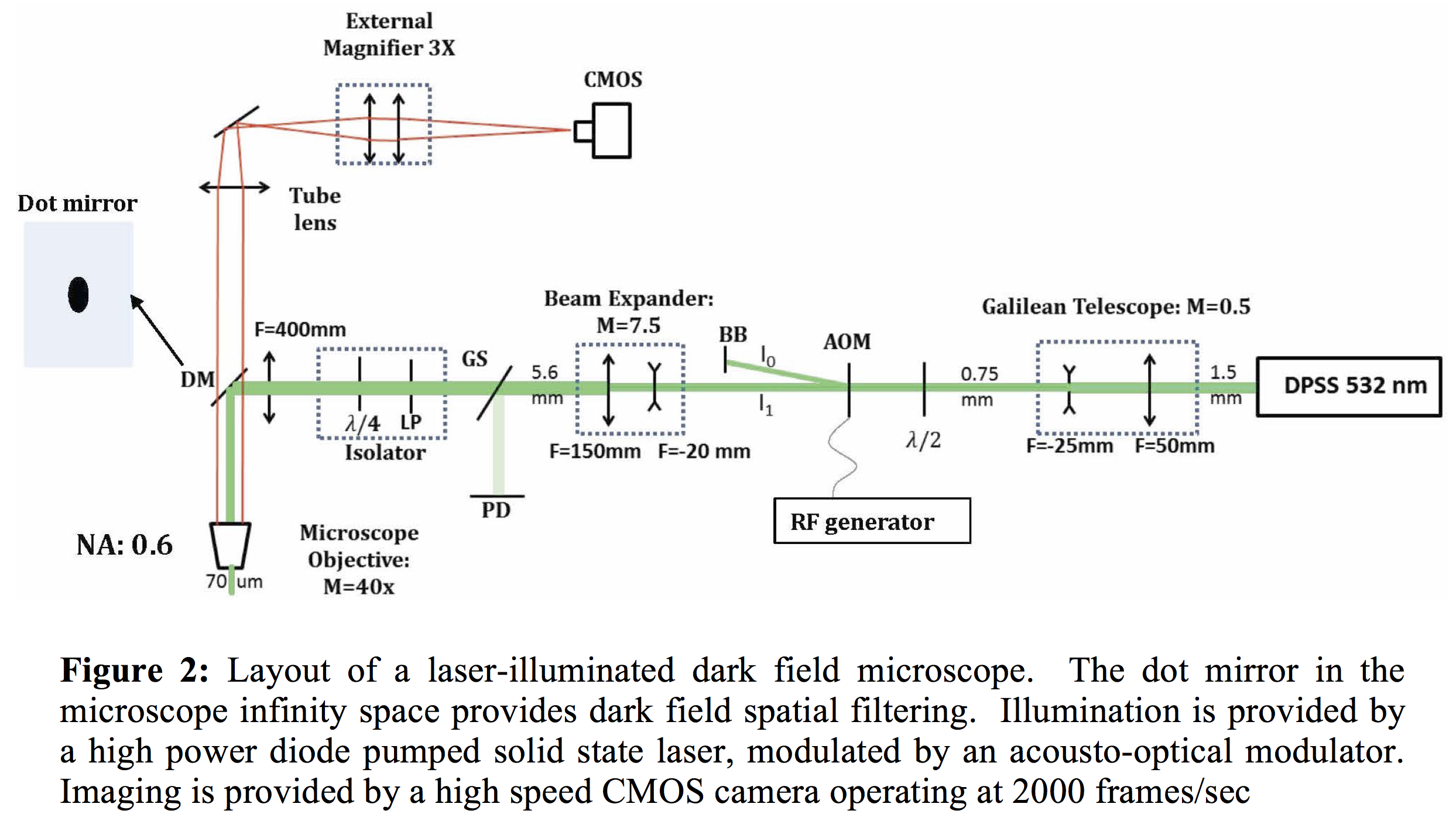
The sensitivity of all interfacial microrheology techniques are ultimately limited by the size of the interfacial probe used; the smaller the better. Imaging small particles for long periods of time (where bleaching precludes fluorescent methods) is best done using laser-illuminated dark-field microscopy, or DFM. Here an illumination laser is sent out of a high magnification microscope objective to illuminate the specimen with a plane wave, and the scattered light from the particles collected by the objective and imaged in the normal fashion. A key component of DFM however is a spatial filter that rejects any plane wave reflections in the system (as from the sample window or the flat liquid-liquid interface), making the background of the image 'dark'. Done carefully, refractile particles as small as 100 nm or plasmonic (gold) nanoparticles as small as 20 nm are readily observable and trackable. To allow high tracking speeds a high power laser is used, enabling shutter times in the µsec range with a high speed CMOS camera, and the laser is strobed to minimize sample heating and photodamage. A schematic of the optical layout is shown in Figure 2, the 'dot mirror' is the spatial filter. In preliminary studies, the microscope performed as expected. We are currently investigating the use of polarimetry to track the orientation of gold nanorods in 3 dimensions, with encouraging results.