Reports: DNI654063-DNI6: Improving Hydrotreating by Tuning Energy Levels
Michele Pavanello, PhD, Rutgers University-Newark
The goal of the project is to investigate electron rich oxide surfaces based on gamma alumina. The focus of the work is, therefore, gamma alumina in its hydroxylated form as well as doped by group V elements, such as phosphorus and/or nitrogen. In the short term, we set out to understand the structure-function relationship of this material with electron-richness being the main guiding paradigm. Theoretical work is ongoing in the determination of electronic properties of gamma alumina.
What was accomplished under this goal?
Major activities
We have set up a high-throughput simulation environment suitable to study a large number of surface configurations of gamma alumina (based on the [001] surface termination of the spinel-like bulk model). We have developed a computational methodology for computing surface-averaged electrostatic potentials and subsequent determination of work functions (WFs) as a function of the oxide termination morphology and chemistry.
We set out to analyze the effects of hydration and hydroxylation of undoped (clean) gamma alumina, including determining the sites of likely dihydroxylation and dehydration, as well as predicting the most stable surface terminations. We have also aimed at including P-atom doping at surface and subsurface sites in the simulations.
Significant results
Non-doped gamma alumina surfaces
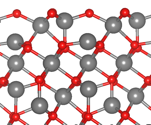
We embarked on a theoretical simulation based on pseudopotential DFT simulations of the [001] surface of gamma alumina in the spinel-like bulk morphology proposed by Gutierrez et al [GutiŽrrez, G.; Taga, A.; Johansson, B. Phys. Rev. B 2001, 65, 012101Ð1], Pinto et al [Pinto, H. P.; Nieminen, R. M.; Elliott, S. D. Phys. Rev. B - Condens. Matter Mater. Phys. 2004, 70, 1Ð11.], and Taniike et al [Taniike, T.; Tada, M.; Morikawa, Y.; Sasaki, T.; Iwasawa, Y. J. Phys. Chem. B 2006, 110, 4929Ð4936.]. The chosen bulk structure compares favorably to the theoretical and experimental results of Paglia et al. [Paglia, G.; Buckley, C. E.; Rohl, A. L.; Hart, R. D.; Winter, K.; Studer, A. J.; Hunter, B. a.; Hanna, J. V. Chem. Mater. 2004, 16, 220Ð236.] A depiction of the 100% hydroxylated surface termination is given in Figure 1 alongside the associated Kohn-Sham DFT Density of States (DOS).
Figure 1: Computed morphology and associated DOS of the 100% hydroxylated gamma alumina surface. The Fermi energy is given a value of zero.
From the figure it is clear that hydroxylated gamma alumina is not electron rich, but instead features the Fermi energy close to the Valence Band Maximum (VBM).
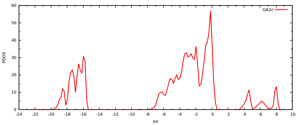
Figure 2: Computed oxygen terminated gamma alumina surface and associated DOS. Fermi energy placed at zero.
In Figure 2 we depict the computed morphology for the oxygen terminated gamma alumina as well as its associated DOS plot. It is clear from the figure that this surface termination activates the material. The unoccupied levels located at the VBM are entirely localized on the surface oxygen atoms, and thus can be utilized for chemical and photophysical applications, however, it is known that this termination is only detectable at very high temperatures. Thus, it is unlikely to play a role in practical applications.
We have studied an array of possible hydroxylation levels, determined surface energies and the most labile OH and H2O groups. We find that no other surface termination, besides the oxygen-terminated one, are electronically activated (i.e. either feature states in the gap or the Fermi energy in either the VBM or CBM). Thus, we set out to compute an array of possible doping configurations of a single phosphorus atom on surface and subsurface cation (Al) sites.
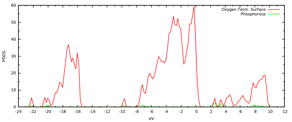
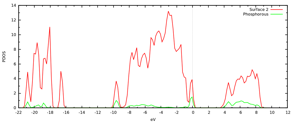
We are in the process of computing an array of possible P-doping sites, either cation or anion doping. We have completed the study of cation doping. In Figure 3 we depict the resulting morphology and DOS of the P-doped gamma alumina. The doping atom was introduced at a tetrahedral Al site. The theoretical prediction delivers a predictable picture: the lone pair of P is donated to the empty states on the oxygen terminated surface. The lone pair states are empty and located in the gap. P assumes a planar configuration consistent with an effective double oxidation.
Figure 3:Computed morphology and associated DOS of P-doped oxygen terminated gamma alumina.
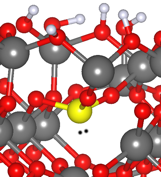
When doping the OH terminated surface, we obtain the morphology/DOS depicted in Figure 4. The P atom is now non-planar, maintaining the lone pair as a partially filled state. This is an important observation, which is also consistent with the fact that the OH terminated surface has no empty states to fill, thus the lone becomes confined on the P atom. And, at the same time, it is sheltered in a sub-surface site.
Figure 4: Computed P-doped OH terminated gamma alumina surface. The lone pair has been added for sake of clarity.