Reports: UNI354419-UNI3: Mechanistic Investigations of Aryl-SO2-Heteroatom Bond Formation from Palladium Using Bench-Stable SO2 Sources
Nicholas D. Ball, PhD, Pomona College
During the 2016-2016 grant year, I was able to support three students with my ACS PRF grant: Ariana Tribby, Melissa Petito, and Cynthia Nyongesa. This narrative will detail the progress made on the specific aims of the funded research. During this year, my research lab has been able to make significant progress one aim 1 involving the studying the insertion of SO2 into Pd(II) model complexes with solid, bench stable SO2surrogates as well as new initiative toward metal-catalyzed generation of sulfonyl fluorides.
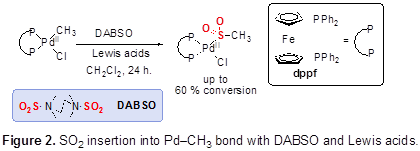
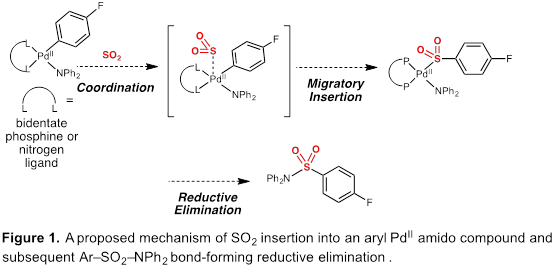
Specific aim 1 focused on synthesizing a series of model Pd(II) aryl amido complexes and studying the mode of SO2 insertion into either Pd–C or Pd–N bonds (Figure 1). Our initial report detailed efforts towards SO2 insertion in to Pd(II) amide complexes; however, the studies demonstrated challenges using gaseous sulfur dioxide. This year we have refocused the aim to demonstrate SO2 insertion can occur with SO2 surrogate DABSO on (Figure 2). We have focused on Pd(II) complexes that have been demonstrated to undergo SO2 insertion with gaseous SO2. Our studies have demonstrated that these complexes indeed undergo SO2insertion with into Pd(II) alkyl bonds using DABSO and Lewis acids with high conversion by NMR spectroscopy. We are currently screening more Lewis and Bronsted acids to increase conversion to the insertion product.
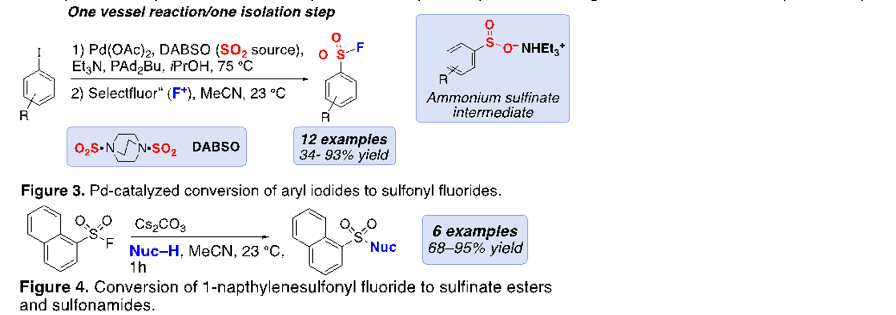
Our work with SO2 insertion with DABSO has led to the development of a new one-pot Pd-catalyzed synthesis of sulfonyl fluorides from aryl iodides. This project is based on recent work in the literature that use Pd-catalysts to convert aryl iodides to sulfones using alkyl halides and DABSO (a solid SO2surrogate). Key to this reaction is an aryl sulfinate intermediate that serves as a sulfur nucleophile to the electrophilic carbon of the alkyl halide. Our approach was to take advantage of the nucleophilic nature of the aryl sulfinate intermediate and use electrophilic fluorinating reagents to make sulfonyl fluorides. Towards this goal, we have developed a one-pot Pd-catalyzed conversion of aryl iodides to aryl sulfonyl fluorides using DABSO and Selectfluor® (an electrophilic source of fluorine) was developed. Electronic and sterically diverse aryl sulfonyl fluorides are generated in modest to high yields (Figure 3). Additionally, we have developed mild conditions to convert sulfonyl fluorides to aromatic sulfonate esters and sulfonamides with aryl amines (e.g., imidazole and pyrazole) (Figure 4). This work has recently been submitted for publication.
Future Directions
We will continue our work on SO2 insertion with bench-stable SO2 sources by expanding to different Pd and Ni fluoride complexes in our on-going efforts to demonstrate C–SO2–F bond formation from metal species. This will be a platform for future metal-catalyzed systems towards the synthesis of sulfonyl fluorides. Concomitantly, we are working on new Pd– and Ni-catalyzed systems to generate sulfonylated compounds with SO2 surrogates.