Reports: DNI754118-DNI7: Effects of Nanoscale Confinement and Electrostatic Interactions on Molecular Conformation of Conjugated Polyelectrolytes
Chuanhua Duan, Boston University
Conjugated polyelectrolytes (CPEs) are π-conjugated polymers containing charged side chains. Because of their high processability and excellent semiconductor electrical/optical properties, CPEs hold great promise for electronics, photonics, energy conversion and biosensing. It is has been widely recognized that the electronic/optic properties of CPEs are determined by their molecular conformations. Unfortunately, conventional methods for CPEs thin film preparation structures suffer from limited control over the molecular conformation and CPEs’ practical applications have been seriously impeded.
In this proposal, we aim to study the effects of nanoscale confinements and electrostatic interactions on the molecular conformation of CPEs, in both the single molecule form and the thin film form. These proposed investigations, if successful, will yield unprecedented insight about CPE structure-property relationship in nanoscale confined spaces and provide new methods to active control CPE conformation.
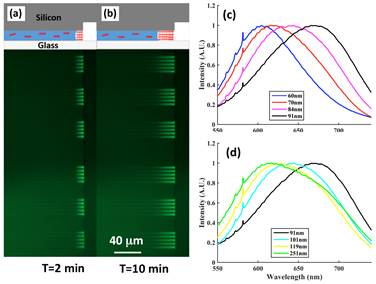
In the first funding period, we prepared permanently sealed silica nanochannels with various heights and used these nanochannels to hold single CPE molecules (in solution) and to prepare CPE thin films via an evaporation-assisted self-assembly method (Fig. 1a&b). We then measured emission spectra of single CPE molecules (MPS-PPV) and CPE thin films in the silica nanochannels. Our results showed the emission spectrum of MPS-PPV thin films exhibited a strong dependence on the silica nanochannel height (Fig. 1c&d), which seemed to confirm the effect of nanoscale confinement on CPE conformation. However, there were two remaining problems. First, CPE thin films formed in the silica nanochannels are permanently embedded and thus cannot be further processed for real applications. Secondly, the CPE thin film formation is a rather time-consuming process due to slow evaporation at the entrance of the nanochannel. In fact, we were only able to form 30-mm-long CPE thin films in 10 minutes (Fig. 1b).
Figure 1 (a)(b) CPE thin film formation in 91-nm-high nanochannels during an evaporation-assisted self-assembly process. CPE thin films formed at the end of the nanochannel while single CPE molecules (in solution) are present in the rest of the nanochannel. Fluorescence images of the CPE thin film (top view) were taken at at t= 2 min and t=10 min. (c)(d) Normalized emission spectra of thin films prepared in silica nanochannels with different heights. The excitation wavelength is 533 nm.
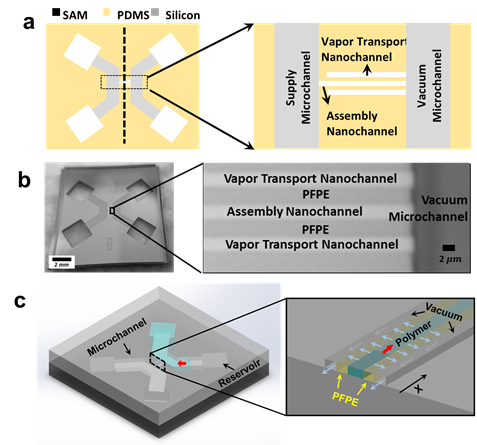
In the second funding period, my students, Yinxiao Li, Mohammad Alibakhshi and I spent most of our time to address these two issues by developing reusable nanofluidic molds which can, a) reversibly bond with the target substrates, b) provide required 2-D nanoscale confinement to control CPE conformation, and c) allow fast solvent evaporation/pervaporation to ensure rapid thin film formation. After many failed trials, we finally developed a new type of hybrid PFPE-silicon nanofluidic molds to achieve this goal. Fig. 2 shows the schematics and the microscopic images of a hybrid PFPE-silicon nanofluidic mold. The top surface of this mold is a photolithography patterned PFPE thin film where a set of nanochannels is defined. This set of nanochannels includes an assembly nanochannel and two parallel vapor removal nanochannels, all of which have a low height-to-width aspect ratio and are separated from each other by a narrow PFPE pervaporation barrier. Among the three nanochannels, the assembly nanochannel is the only nanochannel which has two open ends. Each end of the assembly nanochannel is connected to a microchannel that is created on the underlying silicon substrate. These two microchannels are connected to CP solution and vacuum line through vertical access holes, which are thus referred to as the “supply” and “vacuum” microchannels, respectively. The two vapor transport nanochannels have only one open ends and are connected to the “vacuum” microchannel. To prepare CPE thin film, CPE polymer solution was introduced into the supply microchannel of a nanofluidic mold that was temporarily bonded on a target substrate (Fig.3c). Vacuum was applied to the vacuum microchannel to allow efficient pervaporation of water vapor through the PFPE barireer and vapor removal nanochannels. Using these new reusable PFPE-silicon nanofluidic molds, we were able to prepare CPE thin films with a length up to 1 cm within 10 minutes.
Fig. 2. Reusable PFPE-silicon nanofluidic Mold. (a) Schematics of a nanofludic mold. (b) Microscopy images of a nanofludic mold that is reversibly bonded to a glass substrate. (c) Schematic of the thin film formation process.
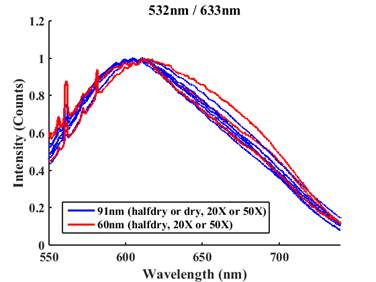
While the PFPE-silicon nanofluidic molds solve the two issues we had in the first year, we found that CPE thin films prepared used these molds did not exhibit the same emission spectra as those prepared in silica nanochannel with the same confinements. In fact, CPE thin films prepared by the PFPE-silicon nanofluidic molds, no matter what the nanochannel height are, exhibit very similar emission spectra (Fig. 3). We are currently still investigating this new puzzle. There are two possible explanations.
1) Despite similar nanoconfinements, the PFPE-silicon nanofluidic molds have much weaker control on CPE conformation than silica nanochannels because of their top hydrophobic PFPE surface.
2) The shift of emission spectrum, observed in silica nanochannels, did not result from CPE conformation change, but some other mechanisms, e.g. interference.
If the first explanation is correct, our proposed active control of CPE conformation using nanoconfinements would still be a promising method to prepare CPE thin films with desired CPE conformation. However, if the second explanation is correct, using confinement and electrostatic force to control CPE formation may not be feasible, at least for the CPE molecules we have tried, i.e. MPS-PPVs with molecule weight ~ 200 kDa. We are currently preparing nanochannels made of other materials and also using CPEs with much larger molecule weight to further test the effect of nanoscale confinement on CPE conformation.
Fig. 3 Emission spectra of MPS-PPV thin film formed in two PFPE-silicon nanofluidic molds with heights of 60 and 91 nm.
In parallel with our investigation of nanoscale confinement on CPE conformation, we also used the stepped nanochannel devices created in the first funding period to measure water transport in single nanochannels and to develop new nanofluidic diodes. Progresses on these two topics have led to two publications in Scientific Reports and Biomicrofluidics, respectively.