Reports: UNI156161-UNI1: New Methods for the Synthesis of Spirocycles via Lewis Acid Catalyzed Dearomative Arene Alkyne Coupling
Paul Vadola, PhD, DePaul University
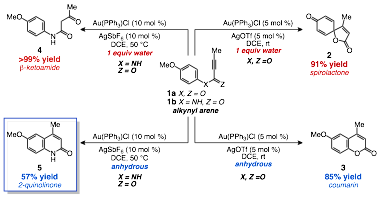
Research efforts in my group focus on the development of new methods for the construction of C—C bonds within the context of heterocycle synthesis. Towards this end we have focused primarily on investigations of catalytic intramolecular alkyne arene coupling, which represents a powerful approach to the synthesis of polycyclic organic compounds. Of particular interest was the potential to develop methods that would allow for the synthesis of both fused bicycles and spirocycles from a common substrate (1a,b, scheme 1). Prior to the award of the ACS PRF UNI grant we had reported on the divergent synthesis of spirolactones (2) and coumarins (3) from aryl alkynoate esters (1a), with the presence of water proving critical to the product selectivity. Following this initial report we sought to further investigate both the mechanism of spirocyclization, as well as extensions of the synthetic method beyond aryl alkynoate ester substrate class. These aims served as the foundation for the UNI proposal.
Scheme 1. Divergent Spirocyclization and Hydroarylation Reactions of Alkynyl Arene Substrates
Following the receipt of the award we began to study the reactivity of N-aryl alkynamides (1b) as potential substrates for spirolactam synthesis. However, when exposed to the standard conditions developed for ester spirocyclization the amide substrates were converted to b-ketoamides via hydration of the alkyne. Removing the water from the reaction mixture led to selective bicycle formation, yielding the 2-quinolinone product 5. While 2-quinolinones were not the intended target of our study a search of the literature revealed that existing methods for the hydroarylation of N-aryl alkynamides relied on the use of stoichiometric harsh Lewis or Brønsted acids. Given the mild nature of gold catalysts we chose to pursue our initial result as a potential neutral method for 2-quinolinone synthesis.
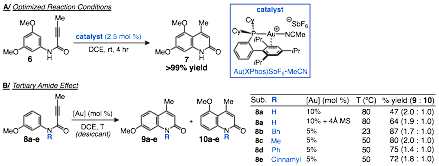
A thorough investigation of gold complexes revealed the commercially available Au(XPhos)SbF6·MeCN to be an exceptional hydroarylation catalyst. Substrates bearing highly electron-rich arenes led to quantitative yields of the 2-quiniolinone products under very mild conditions, room temperature and 2.5 mol % of catalyst (6 to 7, scheme 2). Further investigation of the substrate scope found that arenes of moderate electron density required heating to 50 °C and 5 mol % catalyst loading to achieve complete conversion (8 to 9:10, scheme 2). More interesting was the observation that moderately electron-rich substrates with bearing a secondary amide (8a) were prone to hydrolysis under the established reactions conditions. An increase in catalyst loading, along with the addition of 4 MS and heating to 80 °C led to suppression of the hydrolysis pathway and modest yields (65%). However, the introduction of a substituent on the nitrogen to form a tertiary amide (8b) dramatically improved the reactivity of the substrate, enabling cyclization at 50 °C and 5 mol % with good to excellent yields. The tertiary amide effect was found to be independent of the identity of the N-substituent, with methyl, benzyl, phenyl, and cinnamyl groups all enabling cyclization under mild conditions (8b-e).
Scheme 2. Elucidation of a Highly Efficient Hydroarylation Catalyst and Effect of N-Substitution
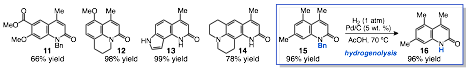
Given the constraint the N-substituent placed on the product scope, specifically the formation of N-substituted 2-quinoinones, we sought to devise a dealkylation strategy that would give access to the secondary amide products. We found that the N-benzyl group could be removed by hydrogenolysis under acidic conditions in excellent yield (15 to 16, scheme 3 insert). In this manner, secondary 2-quinolinone products can be accessed in high yield across the two-step process of hydroarylation followed by hydrogenolysis. The results outlined above have been drafted into a manuscript that is currently under peer review with the American Chemical Society journal Organic Letters.
Scheme 3. Selected 2-Quinolinone Products and Representative Deprotection of N-Benzyl Product
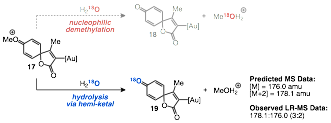
Since the culmination of the amide hydroarylation project we have shifted our focus back towards spirocyclization chemistry. We are currently probing the mechanism of the transformation with particular emphasis on the role of water in the reaction and the impact of alternative activating groups para to the ester substituent on the arene ring. We have run preliminary experiments using oxygen-18 labeled water in our standard reaction conditions (scheme 4). Low-resolution mass spectrometry analysis of the product of the reaction revealed a 3:2 ratio of [M+2]+:[M]+ indicating that approximately 60% of the product contains 18O. In addition, 13C NMR of the same sample revealed two carbonyl carbon resonances of roughly equivalent intensity, which also supports incorporation of 18O. While 18O labeling in the product was not absolute, the results do suggest that the mechanism of demethylation likely proceeds through a hemi-ketal intermediate rather than an SN2-type displacement. We suspect that the incomplete labeling is due to trace 16O-water in the reaction solvent; although commercial anhydrous solvent was employed. Further experiments will employ additional drying techniques to provide more definitive results.
Scheme 4. 18O-labeled Water Leads to Partial Incorporation of 18O into Product
As for the role of the para activating group, we have found that exchanging the p-methoxy group for a p-isopropoxy group leads to a significant drop in reaction rate, which fails to reach complete conversion, and yields a mixture of the spirolactone and coumarin products, along with alkyne hydration. In our original proposal we reported that the introduction of a p-tert-butoxy group allowed for absolute selectivity for spirocycle formation even in the absence of water. Moreover, this transformation could be catalyzed by AgOTf alone. Collectively, these results reveal that the para substituent has a significant role in dictating the product selectivity, reaction rate, and possibly the mechanism. We are currently investigating alternative p-activators and have synthesized a collection of compounds to investigate, some of which feature a departure from the p-alkoxy groups employed thus far in the hopes of accessing new spirocyclic products (scheme 5).
Scheme 5. Investigating Alternative p-Activating Groups