Reports: ND555043-ND5: Anion and Voltage Responsive Crystal Switching at Liquid-Graphite Interfaces for Ion Management in Non-Aqueous Solutions
Steven L. Tait, PhD, Indiana University
Amar H. Flood, PhD, Indiana University, Bloomington
One of the features of supramolecular assemblies that form at dynamic interfaces is the opportunity to develop condensed phase systems that respond to environmental stimuli. The key stimulus studied in this project is the binding of anions in organic solutions, which is an important issue for understanding how to control anions in petroleum and other non-aqueous systems. The focus of this work is on developing a fundamental understanding of the interplay between intermolecular forces that drive assembly and binding interactions. Although the individual forces are understood at a conceptual level, there is not yet a clear understanding of how to treat the interactions of multiple forces in complex systems, such as the monolayer self-assemblies studied here. This ACS PRF grant support has allowed us to make advances in this field by designing, synthesizing, and characterizing families of molecules to probe assembly interactions and anion binding (Figure 1).
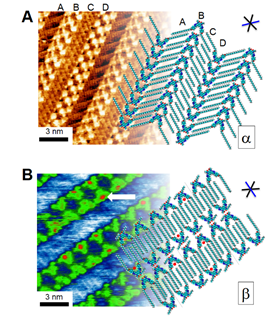
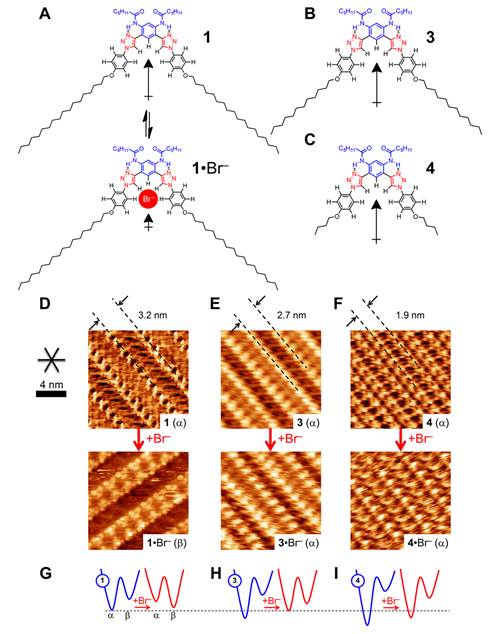
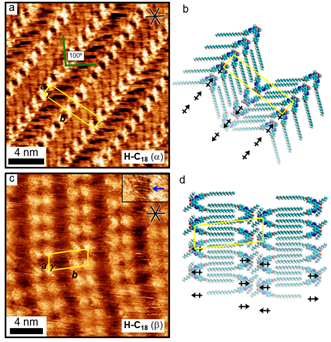
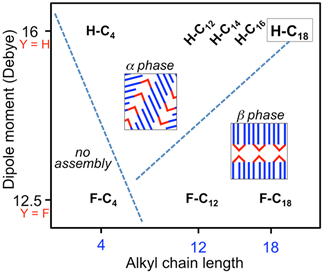
A prerequisite of responsive behavior in supramolecular assemblies is that the supramolecular system be designed to sit very near the stability of two or more crystal structures. We have created such a bi-phasic system with aryl-triazole oligomers (ATO) by investigating how phase morphology is controlled by the interplay between interactions that involve the oligomer’s dipolar cores (Δμ = 3.5 Debye), van der Waals contacts of their pendant alkyl chains (C4 – C18), and close-contact hydrogen bonding. Scanning tunneling microscopy (STM) experiments conducted at the solution-graphite interface allow sub-molecular resolution of the ordered monolayers to unambiguously determine the packing and structure of two principle phases, a and b (Figures 2 and 3). The system is balanced very near the edge of phase stability, evidenced by co-existent phases present over short time frames and by the changes in preference between the two 2D supramolecular assemblies that occur with small modifications to the molecular structure. By conducting an extensive series of experimental studies, we were able to map out a phase diagram of the supramolecular assemblies based on two independent variables that we control at the ATO synthesis stage: alkyl chain length to control influence of van der Waals interactions and substitution on the aromatic system to tune the molecular dipole strength (Figure 4).
We demonstrated that the bi-phasic behavior can be understood as a balance between molecular dipole interactions and van der Waals contacts, two variables within a larger parameter space, allowing synthetic design to move this solution-surface system across the stability boundary of different condensed-phase structures. We constructed a simple theoretical model to develop a more foundational model of these interactions that could be applied to other systems. This model considered electrostatic and van der Waals interactions within the supramolecular assembly based on DFT determination of the atomic charges of a single molecule in vacuum. There were many simplifications and approximations in this model, but it performed remarkably well and was able to reproduce the results in the phase diagram (Figure 4). These findings are a foundation for the development of environmentally responsive 2D supramolecular arrays.
Support from this ACS PRF grant has enabled our development of a better understanding of the interactions between molecules at the dynamic liquid / solid interface. Graduate student Brandon Hirsch was supported by this grant in conducting experimental studies and in developing the conceptual explanations included in the published work. He completed his PhD recently and has gone on to a postdoctoral research associate position at a prestigious university in Europe to advance his career. His success in advancing to the next stage of his career was partially due to the success of the research supported by this grant. Yun Liu was also supported by the grant and has continued to develop a understanding of the synthesis and strategies needed in designing these experiments to allow the project to continue to be successful. For the PIs, this project has been very productive and the advances discussed above in our understanding of the interactions at work in the supramolecular assemblies at dynamic interfaces and opened new research opportunities and stimulated a greater interest by us, and by others in the community, in developing models to describe these interactions and predict phase behavior.
Figure 1. (A) 2D crystal structure of an aryl-triazole oligomer (ATO) with C9 chains determined by scanning tunneling microscopy (STM) real-space imaging with high sub-molecular resolution. (B) STM images and 2D crystal structure of the same ATO self-assembled in the presence of bromide ions (example of anion binding indicated by white arrow).
Figure 2. Chain length has a significant effect on the relative stability of the a and b phases upon anion-induced reduction in dipole strength. (A) Structural representation and dipole strengths of 1 (ATO) and 1•Br–. (D) STM images showing induced phase change from a to b upon Br– addition and binding to 1. Compounds 3 (B) and 4 (C) are designed to have closer inter-row distances but the same change in dipole upon Br– binding. STM images for (E) 3 and (F) 4 show smaller inter-row spacing with concomitant retention of the a phase upon addition of Br–. (G–I) Proposed PES for each compound before (blue) and after (red) addition of Br–.
Figure 3. (a) STM image of the α phase of ATO with C18 alkyl chain (H-C18) at the octanoic acid-graphite interface. (b) Packing model of the α phase that is stabilized by a dipole aligned head-to-tail arrangement. (c) STM image of the β phase observed after deposition of a cooled solution of H-C18 onto a surface at 19 ºC. (d) Packing model of the β phase structure with the head-to-head alignment of the aryl-triazole cores, with dipoles in a clashing arrangment, and nearly “close-packed” interdigitation of alkyl chains along a major axis of HOPG.
Figure 4. Phase diagram illustrating the experimentally observed structural phases for eight aryl-triazole oligomers studied. A simple supramolecular interaction model was constructed based on dipole interactions and van der Waals interactions, which is able to provide theoretical agreement with the experimental data in this phase diagram.