Reports: DNI155734-DNI1: New Chemical Reactions by Engaging Catalytic Ester Enolate Equivalents in Synergistic Catalysis
Thomas N. Snaddon, PhD, Phil, BSc, Indiana University, Bloomington
Overview: This ACS PRF grant has been used to support the development of cooperative catalysis involving C1-ammonium enolates nucleophiles and p–allylpalladium electrophiles. This is a key objective outlined in our original PRF proposal.
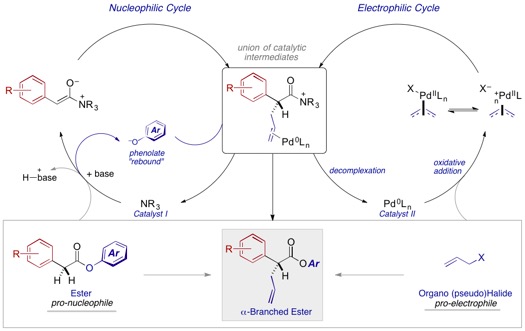
Earlier this year, we published the first direct asymmetric a–allylation of aryl acetic acid esters (J. Am. Chem. Soc. 2016, 138, 5214–5217). Critical to the success of this reaction was the identification and optimization of 'phenolate rebound' as an effective mechanism of catalyst turnover in the nucleophilic cycle (Scheme 1). The scope of this reaction is also noteworthy.
Scheme 1.
While establishing effective reactivity proved challenging, the relevant complications were largely predicted. However, effective control over the enantioselectivity of the process appeared to hinge on the nature of the nucleofuge (X) in the electrophilic cycle. This was not predicted but raises interesting questions concerning both the true role this plays with respect to stereocontrol in the bond-forming event. Remarkably, the bond formation itself proceeds with many different nucleofuges but enantiocontrol varies markedly.
Current Directions: Our efforts in this area has been directed toward three areas:
1. The preparation of stereodefined enolates via methods other than deprotonation.
Here we have accrued preliminary data on decarbonylative processes that exhibit useful levels of enantiocontrol.
2. The effect of Lewis acid catalysts on the stability of C1-ammonium enoaltes
Here we have attempted to stabilize –alkyl C1-ammonium enolates with a variety of common Lewis acids in order to preserve their lifetime at room temperature. This is necessary to permit their reaction with p-allylpalladium electrophiles.
3. A computational collaboration (With Prof. Peng Liu, Pittsburgh University) to elucidate the critical role X– plays on enantiocontrol.
Here we have identified energetically-relevant transition structures.