Reports: ND555659-ND5: Understanding Microscopic- and Molecular-Level Mechanisms Involved in Wettability Alteration on Mineral Surfaces
Udo Becker, PhD, University of Michigan
1. Introduction
The physicochemical properties of mineral surfaces determine their type of wettability, e.g., in oil reservoirs. These reservoirs contain a complex blend of hydrocarbons, aqueous, and gaseous fluids, and the interaction between fluids and reservoir minerals strongly influence reservoir behavior and performance. In particular, a rock reservoir’s affinity to either water or oil, a property known as wettability, is important for oil production and enhanced oil recovery (EOR). Changes between water- and oil-wet states are rarely predictable. Ca2+, Mg2+ and SO42– concentrations and pH of production fluids can influence wettability, and the hydrocarbons themselves are important wettability modifiers, especially the polar components of the heavy fraction (i.e., resins and asphalthenes). Due to the molecular complexity of oil, it is unclear what specific chemical structures or functional groups are directly responsible for wettability changes in the rock, or what steps are necessary to affect this change at the molecular level.
Here, we test what physicochemical properties of the surface change when the wettability change and what electronic properties of the surface are the main driver in wettability alteration. In particular, surface charge density, charge distribution, and the electronic structure determine if the surface is water-wet or oil-wet.
2. Approach
We identify, test, and quantify physicochemical parameters that characterize wettability changes on mineral surfaces. Because surface charge exerts a major control over surface-fluid interactions, changes in the magnitude and distribution of surface potential result from a change in wettability. Calcite, a common constituent of oil reservoirs and generally considered to be water-wet under ambient conditions, is used as the mineral substrate. To induce a change from a water-wet to an oil-wet state, a freshly-cleaved calcite sample was reacted with hexamethyldisilazane ([(CH3)3SiNHSi(CH3)3], HMDS), which can alter calcite wettability from water-wet to oil-wet, as shown through water contact-angle experiments. The microtopography and electrical potential of clean and reacted calcite samples were measured using Kelvin probe force microscopy in order to examine changes in the electronic structure as the result of wettability alteration. We demonstrate that changing the wettability from water-wet to oil-wet on a calcite surface affects the electric potential distribution of the calcite surface. In addition to experimental measurements, quantum-mechanical and classical-mechanical simulations were used to model the adsorption behavior of HMDS and HMDSH+ on different calcite surface sites. The combination of experimental and computational methods provides a more fundamental understanding of the mechanisms controlling mineral wettability and its alteration.
3. Selected results
The adsorption of HMDS and the alteration from a water-wet
to an oil-wet state changes the magnitude and distribution of the electric
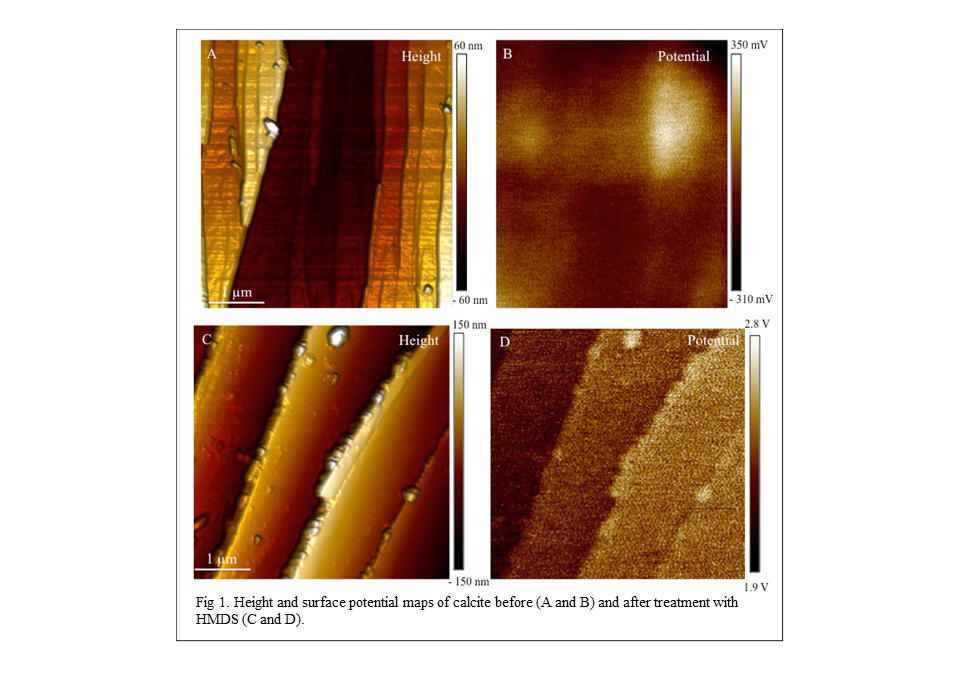
potential on the calcite surface. Surface topography and potential are shown
for the clean and the treated calcite in Figure 1. In the clean sample, there
is no correlation between edges on the surface and potential. In the treated
sample, areas HMDS is adsorbed are more positively charged. This increase in
potential is observed in a second calcite sample also exposed to HMDS (Figure 2).
Discrete HMDS adsorbates, on the order of 10s to 100s of nanometers in height,
are present over the entire surface area of the sample, yet they are
particularly concentrated on step edges on the ![]() surface.
Calcite terraces approximately 10 nm tall are shown in the center of the
height image (Figure 2A), and a well-defined line of hemispherical particles is
visible on the edge of each terrace. These adsorbates appear as bright spots on
the surface potential map in Figure 2B, which indicates that the surface potential
has increased as a result of HMDS adsorption.
surface.
Calcite terraces approximately 10 nm tall are shown in the center of the
height image (Figure 2A), and a well-defined line of hemispherical particles is
visible on the edge of each terrace. These adsorbates appear as bright spots on
the surface potential map in Figure 2B, which indicates that the surface potential
has increased as a result of HMDS adsorption.
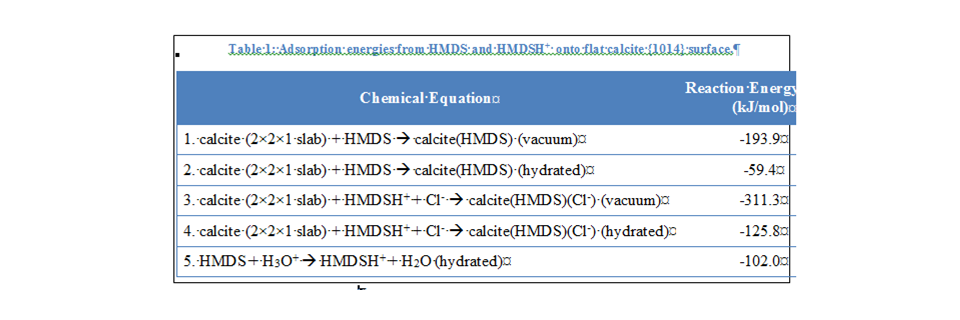
The adsorption energies for HMDS and HMDSH+ on
flat terraces and on edges along the conventional fast and slow
crystallographic directions (![]() and
and
![]() ,
respectively) were calculated in order to compare the adsorption behavior of
HMDS on different surface sites. In order to maintain charge balance between
the two types of adsorbates (HMDS vs. HMDSH+), Cl-
was included in the HMDSH+ calculations on the opposite side of the
slab from the HMDSH+ cation. For the flat
,
respectively) were calculated in order to compare the adsorption behavior of
HMDS on different surface sites. In order to maintain charge balance between
the two types of adsorbates (HMDS vs. HMDSH+), Cl-
was included in the HMDSH+ calculations on the opposite side of the
slab from the HMDSH+ cation. For the flat ![]() terrace,
the reaction energy for HMDSH+ adsorption is on the order of 100 kJ/mol lower
in energy in vacuum conditions and approximately 65 kJ/mol lower in hydrated
conditions, which indicates that this reaction may be more thermodynamically
favorable (Table 1, equations 2 and 4). The protonation of HMDS is also exothermic.
In all cases, adsorption under hydrated conditions resulted in a more positive
reaction energy. This is most likely due to the energy lost from the hydration
of the HMDS and HMDSH+ molecules when moving from the left side to
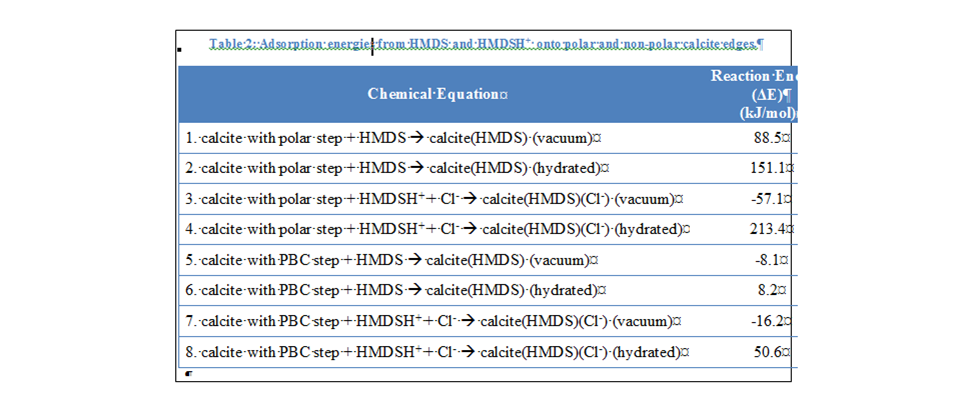
the right side of the equation. For HMDS/HMDSH+ adsorption on the polar and non-polar calcite steps, HMDS adsorbing onto
a non-polar calcite step resulted in the lowest hydrated reaction energy (8.2
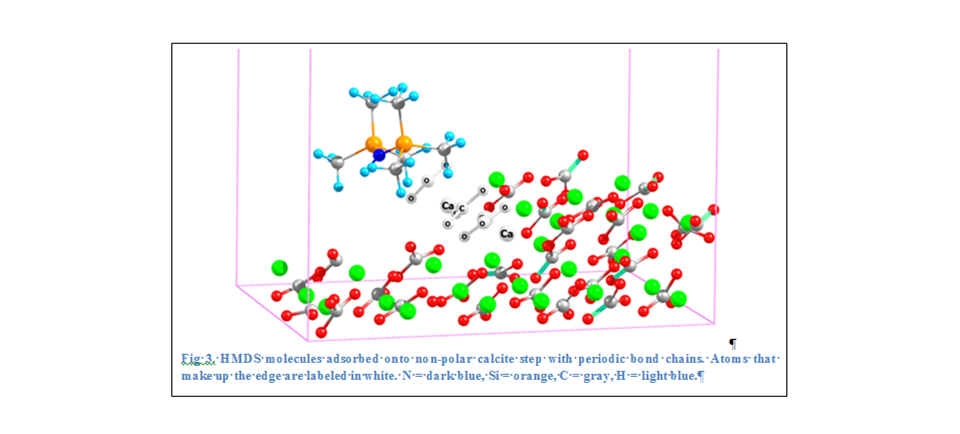
kJ/mol, Table 2). Geometry optimization calculations reveal that the HMDS
molecule flexes slightly; one of the trimethylsilyl groups [Si(CH3)3] bends
towards the surface, and the other group moves away (Figure 3). One side of the
molecule favoring the calcite step may help explain how HMDS alters the
wettability of the calcite from water-wet to oil-wet. HMDS acts as a bridging
molecule between the charged surface and the non-polar hydrocarbon fluid
(similar to a surfactant).
terrace,
the reaction energy for HMDSH+ adsorption is on the order of 100 kJ/mol lower
in energy in vacuum conditions and approximately 65 kJ/mol lower in hydrated
conditions, which indicates that this reaction may be more thermodynamically
favorable (Table 1, equations 2 and 4). The protonation of HMDS is also exothermic.
In all cases, adsorption under hydrated conditions resulted in a more positive
reaction energy. This is most likely due to the energy lost from the hydration
of the HMDS and HMDSH+ molecules when moving from the left side to
the right side of the equation. For HMDS/HMDSH+ adsorption on the polar and non-polar calcite steps, HMDS adsorbing onto
a non-polar calcite step resulted in the lowest hydrated reaction energy (8.2
kJ/mol, Table 2). Geometry optimization calculations reveal that the HMDS
molecule flexes slightly; one of the trimethylsilyl groups [Si(CH3)3] bends
towards the surface, and the other group moves away (Figure 3). One side of the
molecule favoring the calcite step may help explain how HMDS alters the
wettability of the calcite from water-wet to oil-wet. HMDS acts as a bridging
molecule between the charged surface and the non-polar hydrocarbon fluid
(similar to a surfactant).
 |
4.
Ongoing work and career impacts
Changes in the elemental composition of the clean calcite and reacted calcite surfaces will be determined with x-ray photoelectron spectroscopy. Additional KPFM experiments with HMDS and other reservoir-relevant minerals (e.g., quartz and clay minerals) are planned for the near future.
Participating in PRF-funded research has provided a new opportunity for our research group to apply our expertise in surface reactions and atomistic approaches to mineral-liquid interactions to petroleum engineering and hydrocarbon reservoir characterization. While the first year of this work has not led to publications yet, serious method development has been successfully undertaken to characterize wettability as a function of mineral surface, surfactant and liquid composition that can now be harvested for several publications. Among others, this will lead to two publications and two chapters in the thesis of the student involved in this research.











