Reports: ND554130-ND5: Design, Processing, and Characterization of Advanced Binary Nanoparticle Catalysts
Tobias Hanrath, PhD, Cornell University
The ACS New Directions grant has catalyzed several new research endeavors directed at photoelectrochemical CO2 reduction in our laboratory. The majority of the current state of the art in photocatalysis research has focused on understanding and controlling the complex interplay of light-absorption, energy level alignment (i.e., redox potentials) and multi-electron transfer processes involved in photocatalytic CO2 reduction. Future progress along this trajectory risks stagnation unless critical challenges concerning efficient light coupling and mass transport to the catalyst surface are resolved. Towards that goal we sought to establish a better understanding of the interplay between photocatalytic activity, colloidal stability, selectivity, photochemical stability of the core nanoparticles, light coupling, nanoparticle concentration, operating conditions, product recovery, recyclability. Below, we summarize our recent progress towards answering theses questions. Some of the results were reported at the recent annual meeting of the American Institute for Chemical Engineers (AIChE) and will be reported in an upcoming publication.
Photocatalytic activity of Nano Organic Hybrid Materials
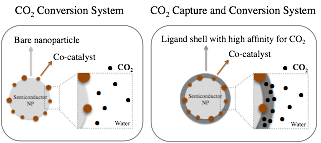
Our approach consists of tuning the surface chemistry of photocatalysts with an organic shell that has affinity for CO2, i.e. ligands with amino groups. Our hypothesis is that this functionalization improves the reaction kinetics by increasing concentration of CO2 near the catalytic sites, and decreases thermodynamic barriers by changing the configuration of the CO2 molecule through chemical and physical sorption (Figure 1).
Figure 1. Comparison between traditional photocatalytic CO2 conversion system vs. our proposed CO2 capture and conversion system.
To test our hypothesis, we have studied the photocatalytic activity of a AuCu-TiO2 nanoparticles for CO2 conversion. We further functionalized these nanoparticles with ligands that contain functional groups with affinity for CO2 to study its impact on performance (Aminopropyl-triethoxysilane and Polyetheramine). Methodology
The methodology consists of the following steps: photocatalyst preparation, surface chemistry tuning by ligand attachment, characterization by means of FTIR and TGA, and performance testing.
Photocatalyst preparation and surface modification.
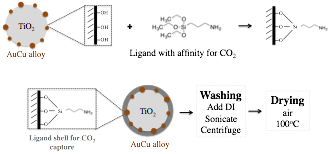
The photocatalyst preparation has been fully described by Neatu et al[1]. We modified the surface chemistry of the photocatalyst nanoparticles by ligand attachmentas depicted in Figure 2. The particles were washed and dried prior to characterization and testing.
Figure 2. Overview of ligand attachment process.
Characterization
We performed FTIR analysis of the synthesized nanoparticles prior and post attachment in order to ensure that we have successfully attached the ligands. In addition, we have characterized the CO2 capture capacity of each sample through CO2 sorption experiments in a TGA.
Performance testing
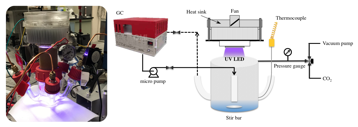
The photoreactor used in our experiments is illustrated in Figure 3.
Figure 3. Working photoreactor (left) and diagram of experimental setup (right). Results and discussion
Characterization
FTIR
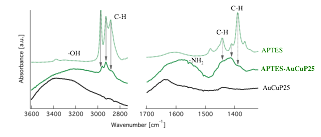
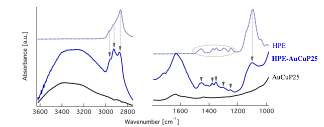
Figure 4 shows the FTIR of the precursor ligands APTES and HPE, sample of functionalized photocatalysts, and photocatalyst prior to functionalization. The characteristic APTES and HPE bands in the functionalized photocatalyst, indicates successful attachment of ligands.
Figure 4. FTIR obtained from ligand precursor (APTES and HPE), functionalized photocatalyst (APTES-AuCuP25 and HPE-AuCuP25), and photocatalyst prior ligand attachment (AuCuP25).
TGA – CO2 sorption
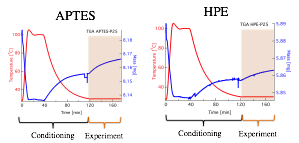
Figure 5 showes the results obtained from the TGA. The orange region in the plot represents the CO2 sorption experiment, i.e. sample exposure to CO2. The increase in mass after CO2 exposure indicates that CO2 is being absorbed by the sample.
Figure 5. TGA plots of sample mass (right axis) and temperature (left axis) over time during the CO2 sorption experiments.
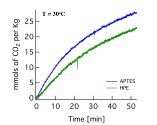
CO2 capture capacity was calculated from the difference in mass prior and post CO2 exposure, and is shown in Figure 6. The absorption of CO2 was approximately 28mmols of CO2 per Kg of APTES-AuCuP25, and 22mmols of CO2 per Kg of HPE-AuCuP25. The greater absorption for APTES-AuCuP25 is likely due to a higher number of ligands attached to the photocatalysts.
Figure 6. CO2 capture capacity of photocatalyst functionalized with APTES and HPE ligands.
Photocatalytic activity testing
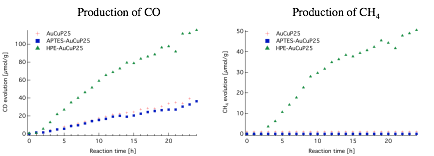
The main reaction products obtained from the photocatalytic activity experiments were methane (CH4) and carbon monoxide (CO). A comparison between the performance of AuCuP25, APTES-AuCuP25, and HPE-AuCuP25 are shown in Figure 7. AuCuP25 and APTES-AuCuP25 had a similar photocatalytic activity, with an evolution of approximately 40umols of CO per gram of catalyst. HPE-AuCuP25 demonstrated better performance for production of CO (115umol/g) and CH4 (50umol/g).
Figure 7. Comparison of the production of CO (left) and CH4 (right) of AuCuP25, APTES-AuCuP25, and HPE-AuCuP25 in umol/g of catalyst.
In addition to CH4 and CO, HPE-AuCuP25 generated C1-C4 products. Conclusions and future work
Photocatalysts were successfully synthesized and functionalized with APTES and HPE. Functionalized photocatalysts demonstrated capability for CO2 capture. Ligand chemistry has shown to play a role in photocatalytic activity.
Further quantitative investigation of ligand coverage will be performed allow direct correlation between CO2 capture capacity, ligand loading, and photocatalytic activity. We will further validate the photocatalytic activity results with (i) control experiments with HPE-AuCuP25 in the absence of CO2, and (ii) experiments with labeled C13.
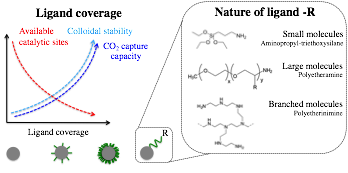
We will Investigate the interplay between photocatalytic activity and colloidal stability. We hypothesize that there is an interplay between photocatalytic activity and colloidal stability. One important parameter is ligand coverage. A possible trend for how ligand coverage may impact availability of catalytic sites, colloidal stability and CO2 capture capacity is depicted in Figure 8.
To test this hypothesis, we will further investigate the photocatalytic activity and stability of different % ligand coverage, according to the methodology described above.
Figure 8. Interplay between ligand coverage and catalytic activity.
[1] Neatu, S., et al. (2014). "Gold-copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water." J Am Chem Soc 136(45): 15969-15976.