Reports: DNI554004-DNI5: Probe Nanoscale Catalytic Sites with Surface-Enhanced Raman Scattering Using Core/Shell Nanoparticles
Jing Zhao, PhD, University of Connecticut
1. Objectives and primary findings:
The objective of the proposal is to develop metal alloy nanoparticles as catalysts and to understand the how the properties of these nanorods depend on their structures. In Pt-Ag allloy system, hollow nanoparticles were formed by thermal treatment included phase segregation and Kirkendall effect. The hollow particles showed high efficiency and stability towards methanol oxidation reaction. In the Au-Cu alloy system, Au-Cu hollow nanorods were obtained by galvanic replacement reaction between AuCu3 nanorods and HAuCl4. The hollow nanorods showed high catalytic activity towards p-nitrophenol reduction.
2. Research Achievements
2.1 Synthesis and Electrocatalytic Activity of Hollow Pt-Ag Nanoparticles
Figure 1. Schematic illustration of hollow Pt-Ag nanoparticle formation, and the corresponding scanning transmission electron microscopy- energy dispersive X-ray spectroscopy images of the nanoparticles during reaction.
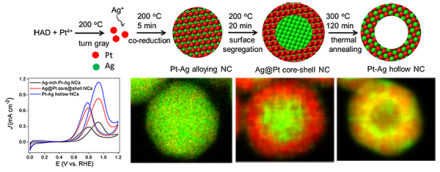
Figure 2. (A) Cyclic voltammetry (CV) profiles of the hollow Pt-Ag nanocatalysts, and Pt/C material in. (B) CV of methanol oxidation on hollow Pt-Ag NCs, and Pt/C. (C) CV profiles of the Pt-Ag hollow nanocatalysts before and after accelerated durability test. (D) Plot of normalized absorbance (I/I0) of p-nitrophenolate at 400 nm as a function of time.
Hollow bimetallic nanostructures exhibit increased durability and utilization efficiency comparing to their solid counterparts, therefore have become promising new candidates for catalytic applications. Here we demonstrate that hollow structured Pt-Ag nanocrystals can be fabricated through a simple one-pot synthesis by employing thermal treatment. During the reaction, Ag-rich Pt-Ag alloy nanocrystals were obtained shortly after co-reduction of Pt and Ag precursors. Subsequent reduction of Pt on the surface of the nanocrystals induced the alloyed Pt atoms to migrate outward to form Ag@Pt core@shell nanocrystals. They finally evolved into hollow alloy structures at an elevated temperature due to the surface energy difference of the metals and Kirkendall effect, as shown in Figure 1.
The hollow Pt-Ag nanocatalysts exhibited substantial enhancement in the current density at 0.9 V and CO-tolerance toward the methanol oxidation reaction, and also showed excellent durability in acid media for methanol oxidation and in alkaline media for p-nitrophenol reduction (see Figure 2). This new method of synthesizing hollow nanocrystals can be used towards the design and fabrication of a wide span of Pt- based bimetallic hollow nanostructures. Future studies include controlling the size of the Pt-Ag hollow nanoparticles and understanding size-dependent catalytic properties.
2.2 Synthesis of Ag-based metal chalcogenide nanorods and their photocatalytic properties
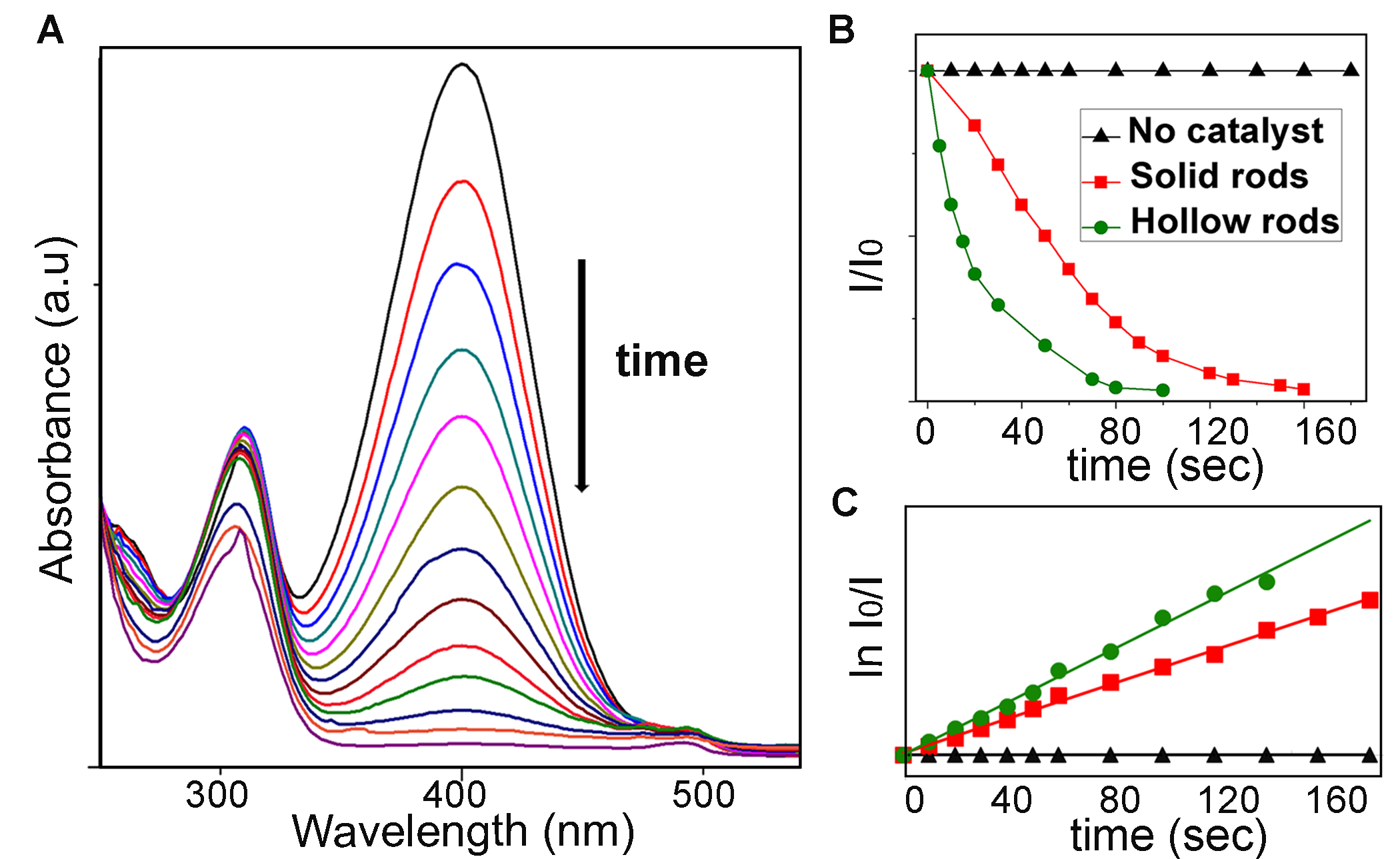
Figure 4. P-nitrophenol reduction reaction. (A) Absorption spectra of p-nitrophenol during reduction with Au-Cu hollow nanorods. (B) Plot of the normalized concentration of P-nitrophenol versus time in the absence and presence of catalysts. (C) Corresponding fitting of the natural log of the normalized concentration versus time.
Figure 3. Schematic illustration of galvanic replacement reaction and the corresponding structures characterized by scanning transmission electron microscopy- energy dispersive X-ray spectroscopy.
span>This study investigates how alloy AuCu3 nanorods transform to hollow rods during galvanic replacement reaction. Galvanic replacement reaction between pre-synthesis nanoparticles and metal salts can generate many interesting nanostructures. Here, AuCu3 nanorods were used as the templates to react with HAuCl4. We followed the reaction pathways with UV-vis spectroscopy, X-ray diffraction and transmission electron microscopy. It is that found that the reaction went through an unusual reaction intermediate where the solid nanorod became partially hollow with Cu rich in one end (Figure 3). This was attributed to the simultaneous galvanic replacement of the Cu from the center of the rod, and the asymmetric diffusion of the Cu to one of the ends due to Kirkendall effect. The difference in the composition of the ends is the driving force for the asymmetric Cu diffusion. The hollow nanorods were shown to be excellent catalysts for p-nitrophenol reduction (Figure 4). The high reduction rate is due to the combined effect of hollowness in the structure, which provides large surface areas, and the availability of Au atoms on the surfaces.
3. Educational Impact:
One postdoctoral scholar, two graduate students, and one undergraduate student were involved in the projects. Postdoc Dr. Shutang Chen performed nanorod synthesis, electron microscopy imaging and X-ray diffraction analysis to study the composition and structure of the nanomaterials. He also performed electrocatalytic measurements of methanol oxidation. Graduate student Sravan Thota carried out the studies of structural transformation of Au-Cu nanorods during galvanic replacement reaction. Graduate student Xudong Wang learned the synthesis of Pt-Ag hollow nanoparticles and tested different experimental conditions. Undergraduate student, Gabriella Reggiano, learned how to synthesize trimetallic nanorod and helped Dr. Shutang Chen to optimize the synthesis. Postdoc and students involved in these projects received interdisciplinary training in nanomaterial synthesis, structural characterization and optical analysis. They also disseminated research findings in scientific publications and presentations in academic conferences.