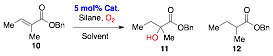Reports: ND154451-ND1: Catalytic Asymmetric Hydration of Alkenes with Chiral cis-β Metallosalen Complexes
Mark Rizzacasa, PhD, The University of Melbourne
Catalyst Synthesis
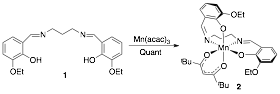
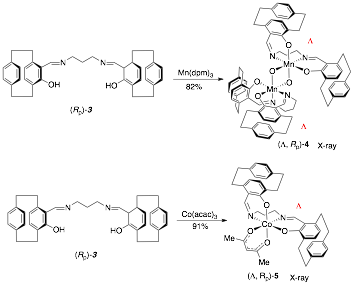
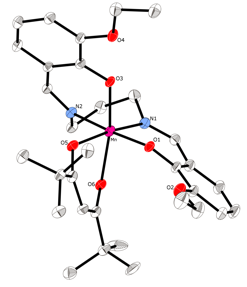
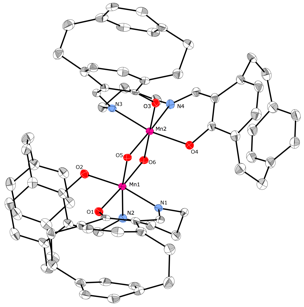
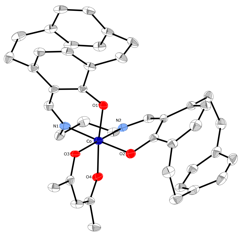
The synthesis of a chiral SALPN catalyst in racemic form is shown in Scheme 1. The SALPN ligand 1 was allowed to react with Mn(acac)3 to give the cis-b octahedral catalyst 2 which was characterised by X-ray crystallography (Figure 1). The OEt-SALPN ligand was key to giving a Mn complex with good solubility and this was then utilized in a number of alkene hydrations as discussed below. Chiral octahedral catalysts were also synthesised as single enantiomers using the [2.2]-paracyclophane ligand1 (Scheme 2). Ligand (Rp) PCSP (3) was allowed to react with Mn(dpm)3 complex in refluxing methanol and crystals of the product obtained from this reaction were subjected to X-ray analysis. Interestingly, the expected L-cis-b complex was not isolated as it rapidly oxidised in air to the subsequent Mn(IV) µ-oxo L, L complex 4 as confirmed by a single crystal X-ray structure as shown in Figure 2.2 Reaction of Co(acac)3 with the ligand 3 gave the complex L-5, again the structure confirmed by a single crystal X-ray analysis (Figure 3) as well as NMR spectroscopy. All the complexes formed as the L isomer only at the chiral metal center as predicted. Thus the ligand (Rp)-PCSP 3 allows for complete control of the asymmetry at the metal center with the L configuration about the metal.2
Scheme 1: Synthesis of Mn SALPN complex 2
Scheme 2: Synthesis of catalysts 4 and 5.
Figure 1: X-Ray structure of 2 (H atoms omitted)
Figure 2: X-Ray structure of 4 (H atoms omitted)
Figure 3: X-Ray structure of 5 (H atoms omitted)
Catalyst Activity
We have tested the reactivity of the new cis-b octahedral catalysts using benzyl crotonate (6) as the substrate with catalyst loadings of 5 mol% (Scheme 3). For comparison, the reaction was conducted using Mn(dpm)3 (8) under the standard conditions with PhSiH3 as hydride source.3 Using catalyst 8 and hexane as a co-solvent gave the hydration product 7 in 20% yield along with a substantial amount of the alkene reduction product 9. With iPrOH as solvent, an improved yield of hydration product 9 was obtained but some reduction product was also produced. The SALPN catalyst 2 gave the desired product 7 in 80% yield using CH2Cl2 as a co-solvent with no reduction product.
Cat. |
Solvent |
Time |
Yield 7 |
Yield8 |
8 |
iPrOH/Hexane |
3 h |
20% |
20% |
8 |
iPrOH |
3 h |
87% |
10% |
2 |
iPrOH/CH2Cl2 |
3 days |
80% |
0% |
Scheme 3: Hydration reactions of substrate 6 using catalysts 8 and 2.
The PCSP complex 4 also gave the desired product 7 in 40% yield but with low ee. Some starting material remained which could not be consumed either by increased reaction times or adding additional reagents. The hydration reaction was then investigated using tiglate 10 as a substrate in (Scheme 4). The complex 8 gave the desired product 11 in 60% yield along with reduction product 12. With the complex 2 as catalyst and the alternative silane Ph(OiPr)SiH2 as hydride source,4 complexes 2 and 8 both gave the desired product along however catalyst 2 did not give nay reduced product 12. To date, no significant asymmetric induction in the hydration of 10 with chiral catalyst 4 has been observed.
Cat. |
Silane |
Solvent |
Time |
Yield 11 |
Yield 12 |
8 |
PhSiH3 |
iPrOH/Hexane |
3 h |
60% |
10% |
2 |
PhSiH3 |
iPrOH/CH2Cl2 |
3 d |
80% |
0% |
8 |
Ph(OiPr)SiH2 |
CH2Cl2 |
3 h |
83% |
10% |
2 |
Ph(iOPr)SiH2 |
CH2Cl2 |
3 d |
80% |
0% |
Scheme 4: Hydration reactions of 10 with catalysts 2 and 8.
These results demonstrate that the SALPN octahedral complexes 2 and 4 are effective catalysts for the hydration of a,b-unsaturated esters and studies are ongoing to see if these are effective in the hydration of other polar alkenes.
References
1. Belokon, Y.; Moscalenko, M.; Ikonnikov, N.; Yashkina, L.; Antonov, D.; Vorontsov, E.; Rozenberg, V., Tetrahedron Asym. 1997, 8, 3245-3250.
2. Loits, D.; White, J. M.; Donnelly, P. S.; Rizzacasa, M. A. Eur. J. Inorg. Chem. 2016, 3541-3544.
3. Magnus, P.; Payne, A. H.; Waring, M. J.; Scott, D. A.; Lynch, V., Tetrahedron Lett. 2000, 41, 9725-9730.
4. Obradors, O.; Martinez, R. M.; Shenvi , R. A. J. Am. Chem. Soc. 2016, 138, 4962-4971.