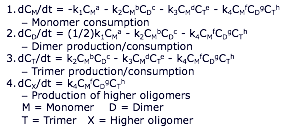Reports: ND754710-ND7: Graphenic Materials from Polycyclic Aromatic Hydrocarbon Oligomers
Mark C. Thies, Clemson University
Introduction. In the previous reporting period, isolation of pure pyrene trimer was reported, along with the discovery that this oligomer is the lowest molecular weight (MW=598) polycyclic aromatic hydrocarbon (PAH) ever found to form 100% discotic, liquid crystalline mesophase. As stated by editor Robert Hurt of the journal Carbon (Hurt and Hu, 1999), 'no unsubstituted, single-component PAH' had ever previously been observed to form liquid crystallinity. Furthermore, the melting point of pyrene trimer was found to be a low 290 °C, as significant melting-point depression occurred because of the multiple isomers of the pyrene trimer that exist.
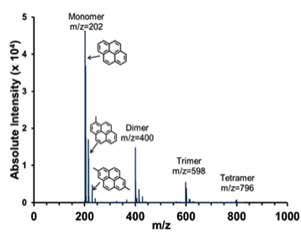
Catalytic Polymerization of Pyrene. For this reporting period, our efforts focused on quantifying how the reaction of pyrene to higher oligomers via catalytic polymerization proceeds so that we could maximize the generation of a given oligomer (e.g., trimer), or of a specific oligomeric distribution (PAH mixtures are also known to form liquid crystalline mesophase). Therefore, experiments were conducted in which pyrene (monomer) was charged to the reactor shown below (see Figure 1), along with 8% AlCl3 catalyst, and polymerized at a constant temperature somewhere between 300 and 400 °C for 1, 4, 8, and 24 hours. A typical MALDI mass spectrum for the resultant polymerized pyrene pitch is given in Figure 2. The monodispersity of the pyrene oligomers is unusual (Sarofim et al., 1994), as the products of catalytic polymerization for PAHs (e.g., naphthalene and anthracene) are typically highly polydisperse due to high levels of fragmentation (Mochida et al., 2000). Furthermore, the MALDI oligomers are actually quite pure, as the peak intensities of the methylated species are ~10x higher than their actual concentration (Burgess, 2010).
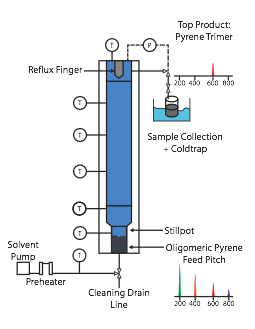
SCE for Oligomeric Make-up of Pyrene Pitch. These pitches were then fractionated in terms of their oligomers with a supercritical extraction (SCE) column setup (see Figure 3), so as to determine the mass of monomer and higher oligomers present in the pitch at each of the above reaction times. (MALDI cannot be used for quantitative analysis as GC-MS can, for example.) Supercritical toluene (Tc=319 C, Pc = 41.1 bar) was used as the extractive solvent, with monomer, dimer, and trimer each being taken off successively as top products in the pure (~99% pure) state at increasing pressures of 20 bar, 36.5 bar, and 52 bar, respectively. Some tetramer could be taken off at even higher pressures, but this and higher oligomers could not be completely extracted, so they remained unextracted in the feed pitch charge in the stillpot (see Figure 3). A detailed description of the process for fractionating pyrene pitch via SCE is given elsewhere (Lamie et al., 2016).
As can be seen for a representative example in Table I, good closure of the overall mass balance was generally obtained, with the error within <0.5-2% at all times. Here we see that the reaction of pyrene for 1 hour generates a pitch consisting of 19.4% monomer, 18.1% dimer, and 6.8% trimer, with the remaining 57.9% consisting of higher oligomers. These values were not obtained from the MALDI, but gravimetrically. MALDI was used only to confirm that a given top product was always pure, and that none of the target 'mers' being extracted at a given pressure were present in the bottom charge at the end of the run. 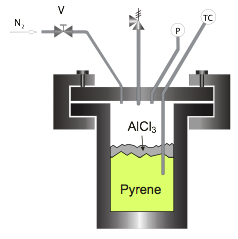
Figure 1. Batch reactor used for the production of pyrene pitches.
Figure 2.
Figure 3. Semi-continuous SCE apparatus for successively extracting pure pyrene oligomers from pyrene pitch; used for composition analysis.
Table I. Supercritical extraction mass balance of 1-hr pyrene pitch from reactor.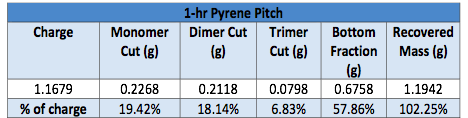
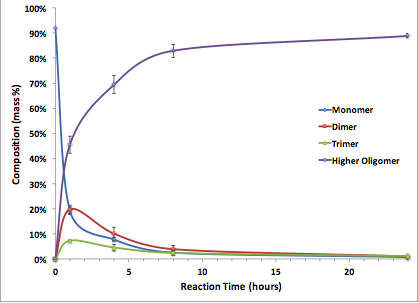
Kinetic analysis. Results of the pyrene pitch fractionation at different time points can be seen in Figure 4. A line of best fit was drawn through the data to help guide the eye and show what trends are occurring. Results indicate that in order to maximize trimer content, the reaction will need to be carried out for relatively short residence times (i.e., ²1 hour). However, as the current batch-reactor setup involves warmup and cooldown periods, the reaction time isn't accurately known. Thus, it may be advantageous to use a continuous plug-flow reactor for future work in order to obtain accurate residence times.
Knowing the mass of the monomer, dimer, and trimer present in the pyrene pitch at 1, 4, 8, and 24 h, we can create a kinetic model for the catalytic polymerization of pure pyrene monomer with AlCl3. We fit the data seen in Figure 4 to a basic reaction-rate model and used a non-linear least squares (NLLS) method to find the reaction rate constants.
Figure 4. Average concentration of pyrene monomer, dimer, trimer, and all higher oligomers (grouped into one point) at 1, 4, 8, and 24 hours of reaction time.
The following system of equations was used to model the system:
The reaction of monomer to dimer was shown initially (0-1 hours) to be a second-order reaction, but it then transitioned to a first-order reaction for longer times. For all reactions except monomer forming dimer, reaction orders were assumed to be 1. This assumption is justified by the fact that most multicomponent reactions are first order with respect to each reactant. Using this assumption, the reaction rate constants were determined to be (using the NLLS method) k1(initial)=0.0457 M/hr, k1(final)=0.4431 hr-1, k2=0.2812 M-1hr-1, k3=0.4726 M-1hr-1, and k4=0.0148 M-2hr-1. However, these are not the final values; the above constants are still being optimized to obtain the best possible fit.
Conclusions. Once optimized, we will use the model to determine the reaction time required to maximize production of a given oligomer or for controlling the oligomeric concentration profile of the overall pitch, depending on the intended use of the material.
Future Work. Work is also in progress for generating PAH oligomers via other techniques not requiring catalyst or elevated temperatures to accomplish the polymerization.