Reports: UR352779-UR3: Triazole-Containing Tridentate Anionic Chelators for Lanthanide and Group VIII Organometallic Complexes
James T. Fletcher, PhD, Creighton University
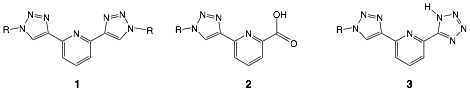
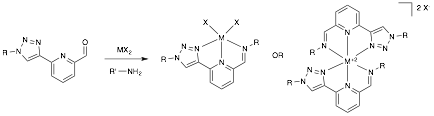
The Click-derived 2,6-bis(1-substituted-1,2,3-triazol-4-yl)pyridine ligand (1) has been shown to coordinate a variety of transition metals in a neutral tridentate manner. The overall goal of this study is to develop two previously unreported 1,2,3-triazole ligand motifs displaying the ability to coordinate transition metals in an anionic tridentate manner: 6-(1-substituted-1,2,3-triazol-4-yl)-2-carboxypyridine (2) and 2-(1H-tetrazol-5-yl)-6-(1-substituted-1,2,3-triazol-4-yl)pyridine (3). Using a click chemistry approach to install the 1,2,3-triazole ring enables the target chelators to be prepared modularly and in high yield, while also allowing a diverse range of functionality to be incorporated onto the periphery of the chelator at the 1-triazolyl position. Functional groups allowing the tuning of electronic properties and solubility are of particular interest, as is exploring the ability of such compounds to form stable 2:1 coordination compounds with Ru(II) and Fe(II) ions and stable 3:1 coordination compounds with trivalent lanthanide ions.
Figure 1. Identity of tridentate chelators 1, 2 and 3.
Year one of this grant established an efficient synthesis for chelators 2 and 3 and identified their ability to form stable, charge-balanced 2:1 coordination compounds with Ru(II) ions. This success was tempered by the fact that many of these Ru(2)2 and Ru(3)2 complexes suffered from poor solubility, limiting the ability to fully characterize these complexes by cyclic voltammetry.
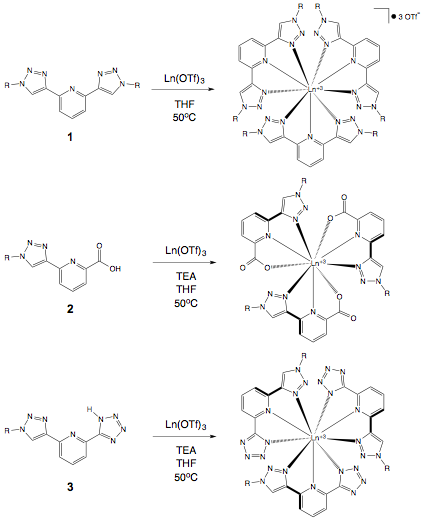
In year two of this grant, attempts to prepare highly soluble analogs of Ru(2)2 and Ru(3)2 complexes were unsuccessful. Stable, charge balanced 2:1 Fe(II) complexes were successfully prepared by reaction of 2 and 3 with FeCl2, although such compounds suffered from the same limited solubility as observed for analogous Ru(II) complexes. Representative lanthanide complexes were also successfully prepared from chelators 2 and 3. Eu(III) and Tb(III) were selected due to their visible and long lived emission properties. The 3:1 chelator:metal stoichiometry and charge balanced nature of the complexes promoted by the anionic tridentate coordination environment was confirmed by X-ray structural analysis.
Figure 2. Preparation of Eu(III) and Tb(III) complexes.
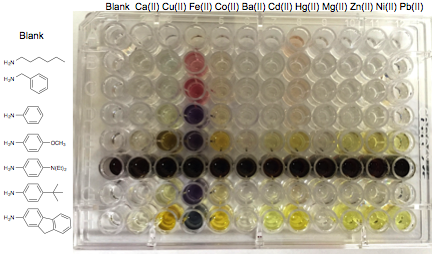
In year three of this grant, a comprehensive series of 3:1 Eu(III) and Tb(III) complexes were prepared in order to determine how coordination environment and peripheral substituent identity each impacted solubility, emission profile and emission lifetime (Figure 2). The majority of these Eu(III) and Tb(III) complexes suffered from poor solubility. Fortunately, the emission properties of such compounds can be measured in the solid state. Samples were deposited in triplicate into a 96 well plate as solutions or suspensions in methanol (Figure 3), and following evaporation were analyzed using a Biotek Synergy H1 multimode plate reader. The fluorescence emission data was observed to be reproducible and independent of the quantity deposited.
Figure 3. Photographic images of Eu(III) and Tb(III) complexes deposited as solids in 96 well plate under room light (left) and ultraviolet illumination (right).
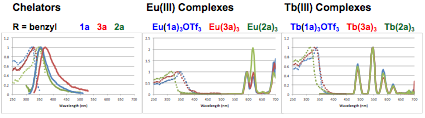
The observed emission spectra (Ex = 250 nm) varied with both coordination environment and peripheral substituents. For most non-aromatic or small aromatic peripheral groups, the emission profile displayed the sharp bands and long lifetimes of lanthanide-based emission (Figure 4). For large aromatic peripheral groups such as anthracene, the emission profile displayed the broad signals and short lifetimes of arene emission. Interestingly, coordination environment and lanthanide identity each also impacted these features. For example, whereas the Eu(III) complex of chelator 3 with peripheral fluorene groups showed lanthanide emission, the analogous complex with chelator 2 showed arene emission. Also, while the Tb(III) complex of 2 with naphthyl substitution showed lanthanide emission, the analogous complex with chelator 3 showed arene emission.
Figure 4. Examples of solid-state emission and excitation spectra.
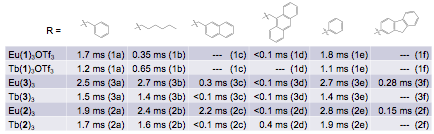
The emission lifetimes observed by plate reader analysis for the Eu(III) and Tb(III) complexes is summarized in Figure 5. In general, the complexes of 2 and 3 showed significantly longer lifetimes relative to 1. For those complexes displaying lanthanide emission bands only, the lifetimes were in the millisecond timeframe and were largely consistent for a given chelator as peripheral substituent varied. For those complexes displaying arene emission bands, the lifetimes were too short to be accurately measured using the plate reader.
Figure 5. Summary of tau values.
Another significant accomplishment in year two of this study was the development of the 2-imino-6-(1,2,3-triazol-4-yl)pyridine motif (4). It was established that such chelators are capable of forming stable 2:1 octahedral complexes with Ru(II) without suffering from solubility issues encountered for chelators 2 and 3. The spectroscopic properties of such Ru(II) complexes were observed to vary subtly with triazole substituent identity and significantly with imino substituent identity.
In year three, the preparation and coordination chemistry of this motif was further investigated. While reversible, this condensation is spontaneous when performed at room temperature in protic solvents such as ethanol. This was exploited in high-throughput screening studies, allowing facile variation of imino substituents while screening for metal coordination events leading to significant spectroscopic changes (Figure 6).
Figure 6. Preparation of complexes from compound 4.
An example of a high throughput screening assay is shown in Figure 7, illustrating the ability of such chelators to reflect metal binding via color changes, and the impact of imino substituent identity on observed spectroscopic properties. Compounds identified from such screening were further investigated by Job plot analysis and mass spectroscopy to verify the binding stoichiometry. Rational synthesis will follow to confirm the identity of the identified complexes.
Figure 7. Assay design and results using a phenyl-substituted triazole chelator analog (1 uM final concentrations in ethanol, 1:1:1 mixtures).
This project was awarded a no-cost extension. Year four effort will aim to complete the required characterization necessary for publication of the Group VIII complexes and the fluorescence emission study. New work will focus on utilizing high-throughput methods to screen imino-functionalized chelators against metal ions in order to discover new leads for colorimetric and fluorimetric chemosensors.
In year three this project has directly supported two undergraduate research students during the 2015-2016 academic year. Presentations describing the results of these studies were made at the 2016 ACS National Meeting in Philadelphia. Manuscripts describing the results of the Group VIII complexes and the lanthanide emission study are currently in preparation and include multiple undergraduate coauthors.