Reports: ND553873-ND5: Reaction Pathways for Methane on Metal Oxide Surface - Influence of Lewis Acidity and Redox Activity
Carsten Sievers, PhD, Georgia Institute of Technology
SCOPE AND PURPOSE
The enormous scale of methane reserves has motivated significant research activities focused on its conversion to fuels and chemicals [1-6]. Since large amounts of natural gas are located in remote areas and transporting gases in pipelines is difficult, processes for producing denser products are desirable. Unfortunately, such processes are challenging. This work addresses the need for a new technology for direct methane conversion by developing catalysts for the selective activation of methane at temperatures below 500 °C.
In the reporting period of this grant, we showed that supported metal oxide clusters add Lewis acid sites (LAS) to redox active ceria-zirconia (CZ). These sites are capable of activating methane at temperatures as low at 150 °C. In case of NiO/Ce0.83Zr0.17O2 (NiO/CZ), in-situ IR spectra showed that methyl groups formed on the surface can couple to longer alkyl chains. The focus of the final year of this grant was on understanding the unique properties of NiO/CZ and realizing the potential of this catalyst for the direction conversion of methane to methanol and higher alcohols.
RESULTS AND DISCUSSION
Catalysts Characterization
EDS maps of NiO/CZ and NiO/SiO2 and NiO/Al2O3 as reference samples showed strong differences in the distribution of Ni on the different supports (Figure 1). The O maps are included to show the dimension of the particles. For NiO/CZ, Ni was highly dispersed, and only few agglomerated particles were observed. In contrast, Ni was only present in large NiO particles on SiO2 and medium-sized, distinct particles on Al2O3.
Figure 1: EDS maps for Ni and O in (a) NiO/CZ (b) NiO/SiO2 (c) NiO/γ-Al2O3.
Further insight into the unique properties of NiO/CZ was obtained by XPS after exposure to methane at 450 oC (Figure 2). After 1 h of exposure, the reduction of Ce4+ to Ce3+ was observed, but there was no reduction of the NiO clusters. When the exposure was extended to 4 h, Ce4+ reduced further and NiO was completely reduced to Ni0. It is suggested that CZ can supply a certain amount of oxygen to keep NiO clusters oxidized. Once this reservoir is depleted, NiO is readily reduced to Ni.
Figure 2: In-Situ XPS of NiO/CZ after exposure to methane at 450 °C (a) Ce (b) Ni
Reactivity Studies
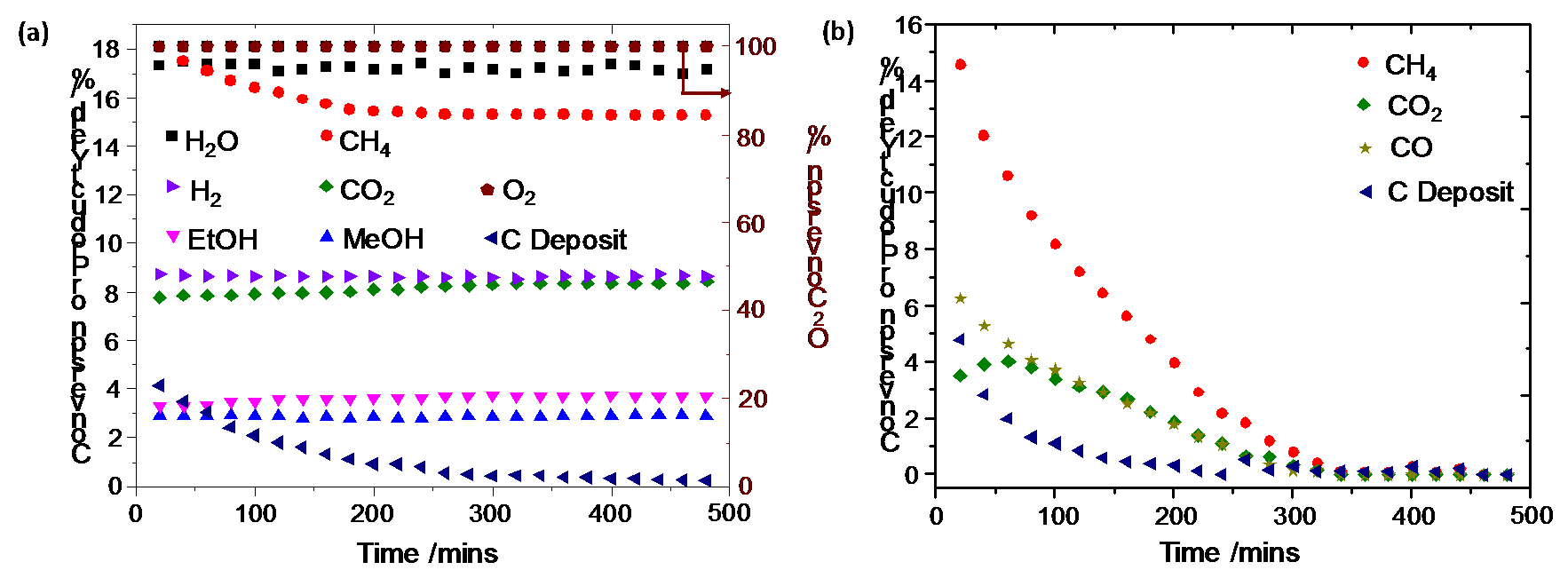
Based on the observation of higher alkyl chains in the IR spectra of surface species on NiO/CZ, we hypothesized that it should be possible to “harvest” these surface species as alcohols by hydrolysis with steam. To provide a thermodynamic driving force for this reaction, O2 needs to be co-fed to oxidize surface hydrogen. We studied the conversion of methane in a packed bed reactor with a feed steam-to-carbon ratio of 1.0, an O2 to carbon ratio of 0.2, and a balance nitrogen (Figure 3a). The initial methane conversion was 18%. It declined to 15% over the first 3 h on stream, but no further changes were observed after that. The partial deactivation is attributed to coke formation on large NiO clusters, whereas smaller clusters are not deactivated. If one assumed that every Ni atom of the catalyst constitutes an active site, the conversion of 15% would correspond to a turnover frequency of 50 h-1. Methanol, ethanol, and CO2 were observed as carbon containing products along with hydrogen and water. The ethanol and methanol yields reached 3.7% and 2.9% on a C atom basis, respectively. This corresponds to a cumulative alcohol selectivity of up to 43%, and an ethanol selectivity of 24% (C atom basis). The yields of methanol and ethanol throughout the reaction remained almost constant. No product with three or more carbon atoms were observed under the conditions applied here. The conversion and selectivity to ethanol are comparable to the performance of typical catalysts for conversion of syngas to ethanol [8]. No alcohols were observed over NiO/SiO2, which only contained large NiO particles (Figure 3b).
Figure 3: Conversion of methane and yields of products formed during reactions of methane, steam and oxygen in a packed bed reactor setup at 450 oC and 1 atm over (a) NiO/CZ (b) NiO/SiO2.
SIGNIFICANCE
Our
findings show that NiO/CZ has great potential for the
production of methanol and higher alcohols from methane in a single reactor. The
results from this grant were the foundation for a proposal to the U.S.
Department of Energy – Basic Energy Sciences that was funded recently (Start
date: October 1, 2016). In addition, several companies have shown interest in
supporting a continuation of this work. The initial submission of these results
to Angewandte Chemie – International Edition was met with interest but also
with skepticism. Additional data collected recently should eliminate these
concerns, and the manuscript will be resubmitted in the coming weeks. In
addition to this communication, we are planning to submit two more
comprehensive articles with foci on non-oxidative coupling and selective
oxidation of methane, respectively. The PI and the graduate student have given
several oral presentations at conferences, which have resulted in strong
interest and engaging discussions.