Reports: DNI654557-DNI6: Tackling the "Fire-In-Ice" Problem in the Petroleum Industry: A Molecular Approach
Sapna Sarupria, Clemson University
Our research focuses on studying heterogeneous nucleation of gas hydrates. Hydrates, or fire-in-ice, are crystalline solids comprising guest molecules entrapped in water cages. The cages are formed through hydrogen bonding between the water molecules and are stabilized by the presence of guest molecules. Various gases such as methane, carbon dioxide, and hydrogen, and heavier molecules like butane, and pentane form hydrates. In the current project, we focus on the effects of surface chemistry on hydrate nucleation. We will study the nucleation of hydrates near hydrophobic and hydrophilic interfaces using molecular dynamics (MD) simulations. We briefly discuss the results from the 1st year of the project and provide details of the activities from our second year of the project.
Brief accomplishments of 1st year of project: In the first year, we successfully designed hydrophobic and hydrophilic surfaces that are compatible with the water model. We are using the monoatomic water model (mW), in which water is represented as a single Lennard Jones sphere. The tetrahedrality from the hydrogen bonding between water molecules is imposed as a three-body potential in the Hamiltonian. Because the model does not include electrostatic interactions and hydrogen atoms, it speeds up the simulations by a factor of 5. The ability to sample long simulation times is necessary for studying nucleation. Since mW model is relatively new, limited parameterization to characterize the interactions between different solutes and water have been developed. Therefore, our first step was to develop potentials to represent hydrophobic and hydrophilic self-assembled monolayer (SAM) surfaces in our simulations.
Our second focus was on developing and setting up calculations to determine the rate of sII hydrate nucleation and elucidate on the underlying mechanisms for sII hydrate nucleation. Nucleation is a rare event meaning that the timescales of observing nucleation events are longer than the simulation timescales. To address this challenge we use an advanced sampling technique called forward flux sampling (FFS), in which the sampling of the transition from state A to state B is enhanced by dividing the transition into multiple stages and sampling each stage extensively. We have developed software called Scalable FFS (ScaFFS) that performs large-scale FFS calculations efficiently in high performance computing environment. In Year 1 we focused on validating the software and ensuring that the parameters chosen for hydrate nucleation calculations were appropriate.
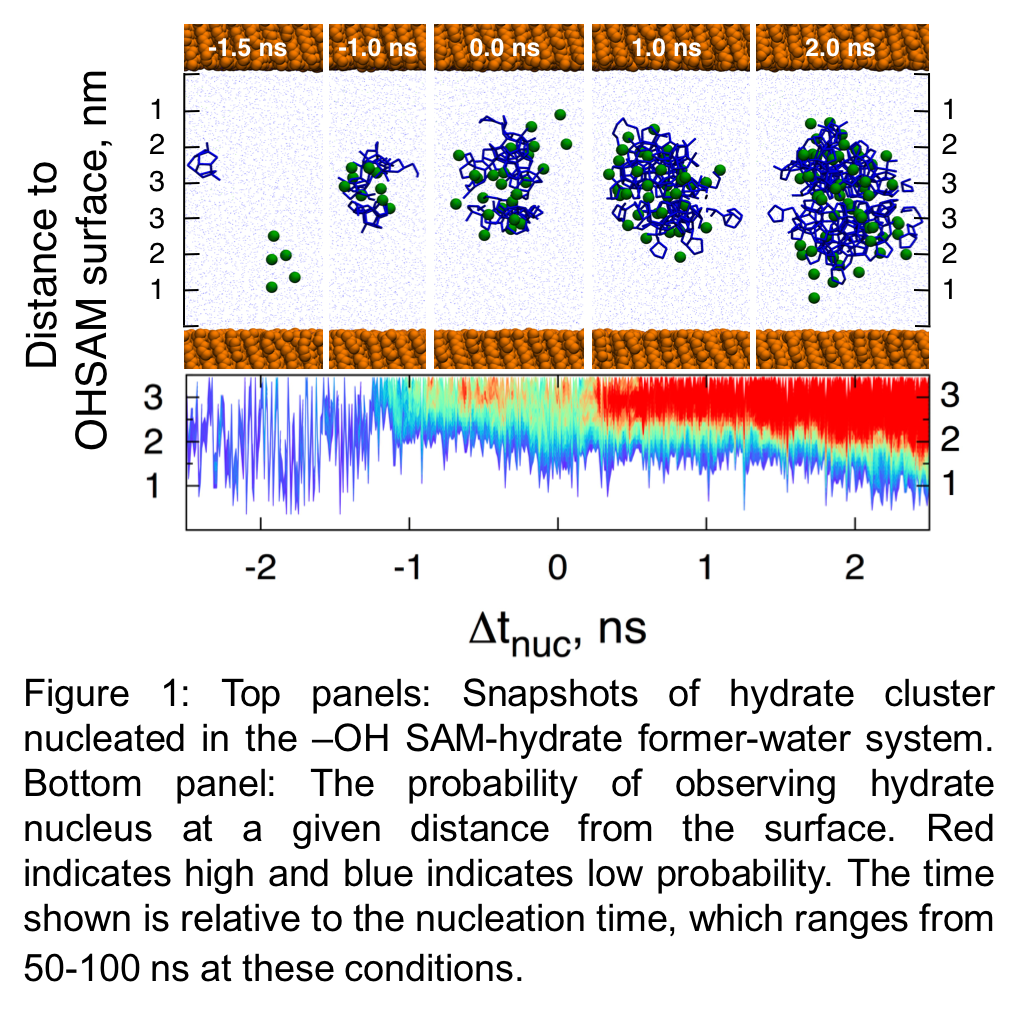
Accomplishments of 2nd year of project: In the second year of the project, we have performed large scale MD simulations of solutions of sII hydrate former and water in contact with the hydrophobic and hydrophilic SAM surfaces. We are investigating the effects of temperature, pressure and solute concentration on the surface effects observed in our simulations. In total we have performed 30 simulations of at least 250 ns each, and longer in cases where no nucleation was observed, resulting in more than 7.5 microseconds of simulations. We are currently performing analysis of this simulation data to elaborate on the role of surface chemistry in affecting the nucleation rate and mechanism. Our preliminary analysis indicates that hydrate nucleation occurs few water layers away from the SAM surfaces indicating that the surfaces do not directly participate in hydrate nucleation (see Fig. 1). We observe that hydrates nucleates faster in the presence of the hydrophilic SAM surface relative to the hydrophobic SAM surface. We hypothesize that this is due to the difference in the behavior of the guest former near the two surfaces and our on-going analysis is investigating this further.
We have performed large scale FFS calculations of homogeneous sII hydrate nucleation. These calculations involved more than 250,000 simulations, and represent the largest scale of FFS calculations reported so far for hydrate nucleation. From these calculations, we will calculate the rate and elucidate the mechanism of hydrate nucleation. We will identify the reaction coordinate that best captures the transition from sII hydrate former-water solution to sII hydrate structure. Preliminary analysis of the data indicates that previously used order parameters such as largest cluster size of hydrate-like water molecules and largest cluster of mutually coordinated guest molecules are inadequate to describe the complete hydrate nucleation process. These studies are on-going and will be the basis for performing similar studies of heterogeneous nucleation.
Our studies have also resulted in new directions for the project. Based on our experience with the water model, we have concluded that using coarse grained models to study heterogeneous hydrate nucleation is good for model surfaces but limits the interaction space that can be investigated. For example, tetrahydrofuran (THF) is a sII former which is represented as a sphere (with no hydrogen bonding) in the coarse grained model. However, hydrogen bonding of THF with water and the surface is expected to play a role in hydrate nucleation. This is not captured in the coarse grained models and therefore, we will use all-atom models to investigate these contributions to hydrate nucleation.
The project involves one graduate and two undergraduate students. This work, made possible through the ACS PRF grant, has provided the graduate student and the PI opportunities to participate in conferences. This has helped in enhancing their professional network and research experience. The PI presented at the PPEPPD 2016 conference in Porto, Portugal. The involved graduate student will present the work at the AIChE National Meeting in November 2016. The undergraduate students have received training in basic concepts of gas hydrates and performing molecular simulations – both of which are not available in the traditional chemical engineering curriculum. The results from this work have been used as preliminary results for other grants.