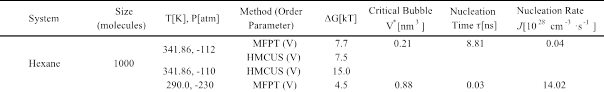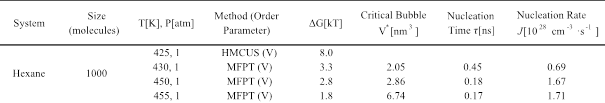Reports: ND654372-ND6: Molecular Simulation of Vapor-Phase Nucleation of Alkanes
Fernando A. Escobedo, Cornell University
Summary
The main goal of this project is to study via molecular simulation the nucleation of vapor-phase bubbles from metastable liquids, especially of alkanes under negative pressures. We used hybrid Monte Carlo umbrella sampling (HMCUS) scheme and Mean first passage time (MFPT) technique to calculate the free energy barriers to homogeneous liquid-vapor nucleation in the superheated and over-stretched hexane and hexane-methane mixtures. We tracked the largest bubble and use its volume as a local order parameter to describe the nucleation process. The free energy barriers obtained using HMCUS are in agreement with those found using MFPT for cases when spontaneous nucleation occurs. Expectedly, it is found that the bubble nucleation free energy barriers decrease with increasing degree of superheating and of super-stretching. The cavitation pressures of metastable hexane liquid obtained in our simulations are in accord with both the reported limits of stability (spinodal points), and with predictions of classical nucleation theory (CNT).
We made progress in 3 fronts; applications to: (i) pure n-hexane, (ii) a mixture n-hexane and methane, (iii) heterogeneous nucleation in pore confinement.
1) Pure hexane
We investigated two types of metastable conditions for liquid hexane: super-stretched states at P = -112 and -110 atm at T = 341.84 K, and super-heated states at T = 425, 430, 450 and 455 K at P = 1 atm. The main results for the super-stretched conditions are given in Table 1.
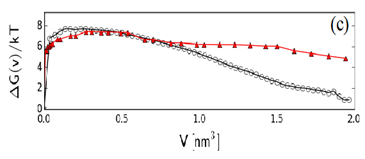
For T = 341.86 and P=-100 atm, the calculated free energy barrier found via the HMCUS method is DG = 15.0 kBT. To access spontaneous nucleation events (suitable for the use of the MFTP method), we simulated the system at 341.86 K and -112 atm and used both HMCUS and MFPT to cross-validate their results. The DG found using both methods (see Fig. 1) was the same, ~ 7.5 kBT, although the shape of the free energy profile shows some inconsistencies for large bubble sizes, likely due to the scarcer statistics available for the application of the MFPT method. At these conditions, the system is not far from the limit of stability (DG » 1 kBT), which is in line with experimentally found cavitation pressure of -119 atm at 342 K for over-stretched heptane.
The main results for the super-heated states are given in Table 2. At T = 425 K and P = 1 atm, bubble nucleation did not happen spontaneously and hence we used HMCUS scheme to get DG ~ 8 kBT. To explore the limit of stability of liquid hexane at 1 atm, we performed simulations at 430, 450 and 455 K using the MFPT method. The barrier to nucleation was ~ 1.5 kBT at 455 K, consistent with the experimentally determined cavitation temperature of 457 K at 1 atm.
2) Hexane-Methane mixture
We performed simulations for mixtures with various compositions. For any composition, there is only a narrow range of temperature where the two species can exist as a liquid; i.e., between 178 K (triple point of hexane) and 190.6 K (critical point of methane). We first explored the limit of metastability for the super-stretched mixture with 10% methane. At 290 K and -190 atm, we used the MFPT method to get DG ~ 3.9 kBT, a value that is not far from the results of metastable pure hexane liquid whose cavitation pressure was -230 atm at 290 K. In comparison to pure hexane at the same conditions, as the composition of the more volatile molecules (methane) increases, DG decreases.
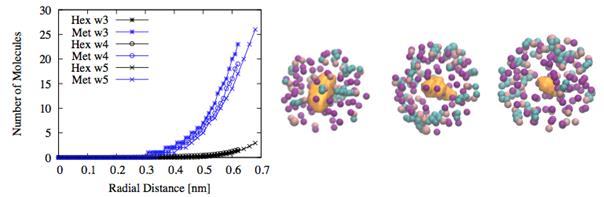
Our analyses reveal the presence of non-spherical bubble shapes and a non-homogeneous population of hexane and methane molecules as a function of the radial distance from the center of the bubble (see Fig. 2). Expectedly, the bubble cavity is predominantly surrounded by methane molecules. This phenomenon is due to both a thermodynamic driving force (disparity in volatility) and kinetic effects (disparity in diffusivity).
3) Heterogeneous bubble nucleation in nanoscopic pores
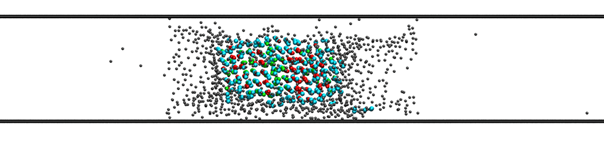
Phase transitions involving vapor, liquid, and solid phases at very low temperatures is important to processes occurring in aerosol particles at high altitudes in the atmosphere. Such particles contain very small pores where liquid and even solid phases are metastable with respect to the vapor phase. We are first focusing on water as the primary substance of interest, to leverage ongoing research of a collaborator. Similar studies will be conducted later on for methane and propane as prototypical alkanes in the environment. The goal is to map out temperature-pressure conditions where liquid metastability ensues inside nanoscopic pores and to elucidate the microscopic structure of the metastable fluid as it vaporizes (see Fig. 3).
Impact
A doctoral student, Sai Pooja Mahajan, was initially involved with by this project and graduated by the end of 2015. During 2016, Endian Wang, a Master of Science, has been working on this project as a temporary worker and will be applying to our Ph.D. program.
Table 1. Free energy barrier DG, nucleation time, critical bubble volume V* and nucleation rate J for over-stretched hexane.
Table 2. Free energy barrier heights DG, nucleation time, critical bubble volume V* and nucleation rate J for superheated hexane.
Figure 1. Bubble nucleation free
energy profile for hexane at 341.86K and -112 atm using the MFPT method
[circles] and HMCUS method [triangles] as a function of the largest bubble
volume V. The maximum in the curve identifies the free-energy barrier height DG and the critical
bubble volume V*.
Figure 2. Distribution of
methane (blue) and hexane (black) in nucleated bubbles at 184 K, -230 atm.
Left: Number of molecules as a function of radial distance from bubble center.
Right: Picture of the bubble within r = 0.8 nm from the center. [Color
code: bubble (yellow), methane (purple), hexane (CH3 pink and CH2
cyan)].
Fig. 3. Illustration of metastable water
inside a nanoscopic cylindrical pore (of 2 nm raadius) in contact with a stable
vapor phase at T=205 K and 10 Pa. Color code: Red: cubic ice, Green: hexagonal
ice, Cyan: interfacial ice, Gray: liquid and vapor water.