Reports: UR1055521-UR10: Electrochemical Interactions between Conjugated Polymers and Polymerizable Salts
Janelle M. Leger, PhD, Western WA University
Characterization of structural changes:
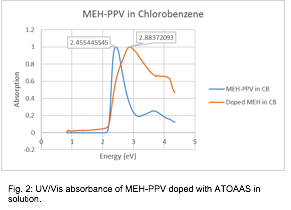
Fig. 2: UV/Vis absorbance of MEH-PPV doped with ATOAAS in solution.
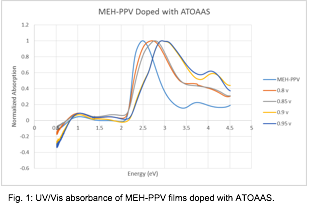
Fig. 1: UV/Vis absorbance of MEH-PPV films doped with ATOAAS.
We have proposed a number of characterization techniques that aim to identify the nature of the electrochemical interaction between the polymerizable ionic liquid (PIL) ATOAAS and a conjugated polymer (CP). Our first focus was on reproducing a study in which mid-bandgap energy levels were identified using UV/Vis absorbance. In MEH-PPV, these additional energy levels are identified by the addition of two low-energy absorbance bands in doped films. We have attempted to identify these bands in MEH-PPV doped with ATOAAS, and in the process have encountered some unexpected results. Specifically, as we increase the doping potential in an MEH-PPV film using the dopant ATOAAS, the overall bandgap shifts from 2.45 eV to 2.88 eV, with the creation of two mid-band gaps around 1.0 eV and 1.81 eV (Figure 1), similar to doping with a non-PIL. However, these mid-band gaps disappeared when the fully doped slides are dissolved into a chlorobenzene solution. The relative shift in the overall band gap remains the same, but we no longer observe the two mid-band gaps (Figure 2). Therefore, it is unclear whether the material is remaining doped upon dissolution.
Gel Permeation Chromatography (GPC) was used to characterize the shift in molecular weight distribution between undoped and doped MEH-PPV. MEH-PPV was doped to 0.9 volts, dissolved in Tetrahydrofuran (THF) and run through a Phenogel 10000A column. We compared the distribution of undoped MEH-PPV with doped MEH-PPV. We expected the distribution of the two samples to either be indistinguishable, due to MEH-PPV's large distribution of molecular weights, or a slightly higher molecular weight associated with the doped sample due to the addition of allylsulfonate during the doping process. However, we found that the unmodified MEH-PPV has a peak molecular weight of 1 million and a polydispersity index (PDI) of 4.3. The doped sample of MEH-PPV has a peak molecular weight of 9 k and a much narrower range of molecular weights (PDI to be determined). These unexpected results lead us to believe that the reaction is significantly more complicated than originally expected. This will be a significant focus of our upcoming funding period.
Novel ionic liquids:
Two new PILs were successfully synthesized in collaboration with Greg O'Neil (WWU Chemistry), ATMAOS and VTMAOS (Figures 3 and 4). This was accomplished through the development of a new synthetic strategy that avoids some of the challenges (e.g. avoidance of inorganic salts, vide infra) associated with ATOAAS. The products were pure by NMR. For this application, we are also very concerned with inorganic salt impurities, therefore we also test the materials using Flame AA and HPLC. Results of these tests indicated acceptable levels of Na (2-4 ppm), Ag (<1 ppm), and Br (2-4 ppm) in both products. Moderate
Fig. 3: ATMAOS (above) and VTMAOS (below).
Fig. 4: New PILs synthesized.
amounts of Cl were detected in the VTMAOS (8 ppm) but acceptable amounts in the ATMAOS (<2 ppm). When blending our conducting polymer (MDMO-PPV) with the PILs, some issues with compatibility became apparent. Figure 5 shows an atomic force micrograph of MDMO-PPV films blended with ATMAOS at relatively high loading and following an anneal step. Clear phase separation is evident as islands of ATMAOS can be observed protruding from the polymer film at several hundreds of nanometers high (the film thickness is 200-300 nm). This problem has been mitigated somewhat by using lower loading of ATMAOS in the polymer and by avoiding the annealing step. However, the problem is still present. Phase separation with VTMAOS appears to be less severe, but still present. Early attempts have been made to determine whether an alternative polymer to MDMO-PPV could be used that has better phase compatibility with ATMAOS and VTMAOS. So far we have not found an appropriate substitute to MDMO-PPV, but more work remains.
We have performed cyclic voltammetry of MDMO-PPV films using an electrolyte based on ATMAOS and VTMAOS. This has presented some challenges as these materials are not completely soluble in acetonitrile (the typical solvent used in these experiments). However, basic results could still be obtained for ATMAOS; the expected loss of reversibility of doping was easily observed. For VTMAOS, results could not be obtained because the Cl- contamination prevented the preparation of a suitable reference electrode (the electrolyte must be mixed with silver nitrate – in the presence of Cl- an undesirable precipitate forms). This will be mitigated by further purification in future efforts.
Finally, we have incorporated both ATMAOS and VTMAOS into polymer fixed junction photovoltaic devices. VTMAOS devices were not successful, likely due to the presence of Cl- impurities that cause an incomplete fixing of the pn junction. In all devices, at high loading of salts, significant device shorting was observed likely due to the phase separation described above. However, at lower salt loading, we were able to observe a fixed junction and photovoltaic response in the ATMAOS devices. We are currently in the process of optimizing the device fabrication procedure to improve performance.
Overall outcomes:
While our work has not resulted in publications so far, we have made important progress scientifically, and there have also been important outcomes in terms of student preparation. Two undergraduate students, James Bauman and Nick Whitcomb, were given the opportunity to carry out full time research during Summer 2016, as well as part time research during the 2015/16 academic year. James will continue with his research during the 2016/17 academic year as well. In addition, I will take on one more undergraduate student this year. I also have a new Chemistry M.S. student who is beginning work related to this project (Sarah Clark).