Reports: ND155220-ND1: Strained Cyclic Allenes: Novel Methods for Generation, New Trapping Reactions, and Domino/Cascade Processes
Frederick G. West, University of Alberta
This project seeks to develop new, mild methods for the generation of high-energy strained cyclic allenes, and to discover efficient, new reactivity driven by strain relief. In particular, we are interested in intercepting the reactive allenes via pericyclic processes, allowing for the establishment of multiple new strategic bonds and stereogenic centers. The combination of efficient generation methods and novel trapping modalities will then allow the application of this chemistry in the synthesis of complex target molecules.
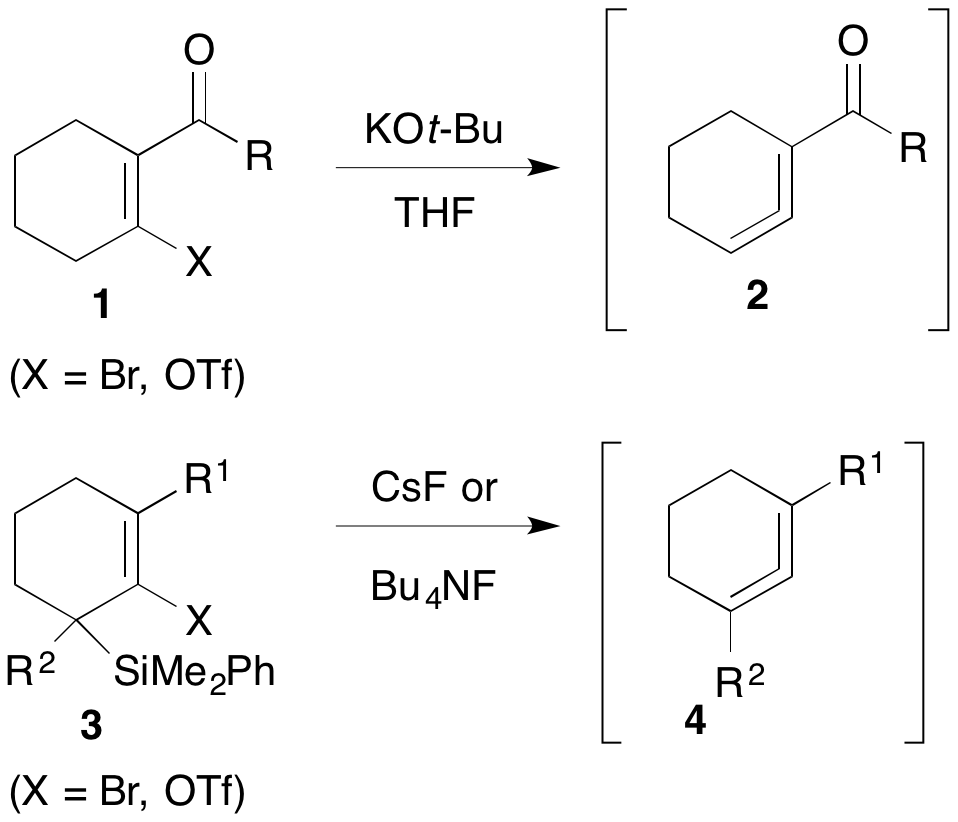
So far, we have explored two approaches for allene generation, both involving elimination of leaving groups situated on sp2 alkene carbons. Ester- and ketone-substituted cyclohexenes 1 undergo efficient elimination of the 'X' group (typically Br or OTf) upon treatment with potassium tert-butoxide to form electron-deficient cyclic allenes 2 (Scheme 1). On the other hand, readily accessible allylic silanes 3 bearing alkenyl leaving groups on the carbon adjacent to the silyl group are cleanly converted to electron-neutral cyclic allenes 4 when treated with fluoride sources. Both methods are tolerant of other substituents on the ring.
Scheme 1
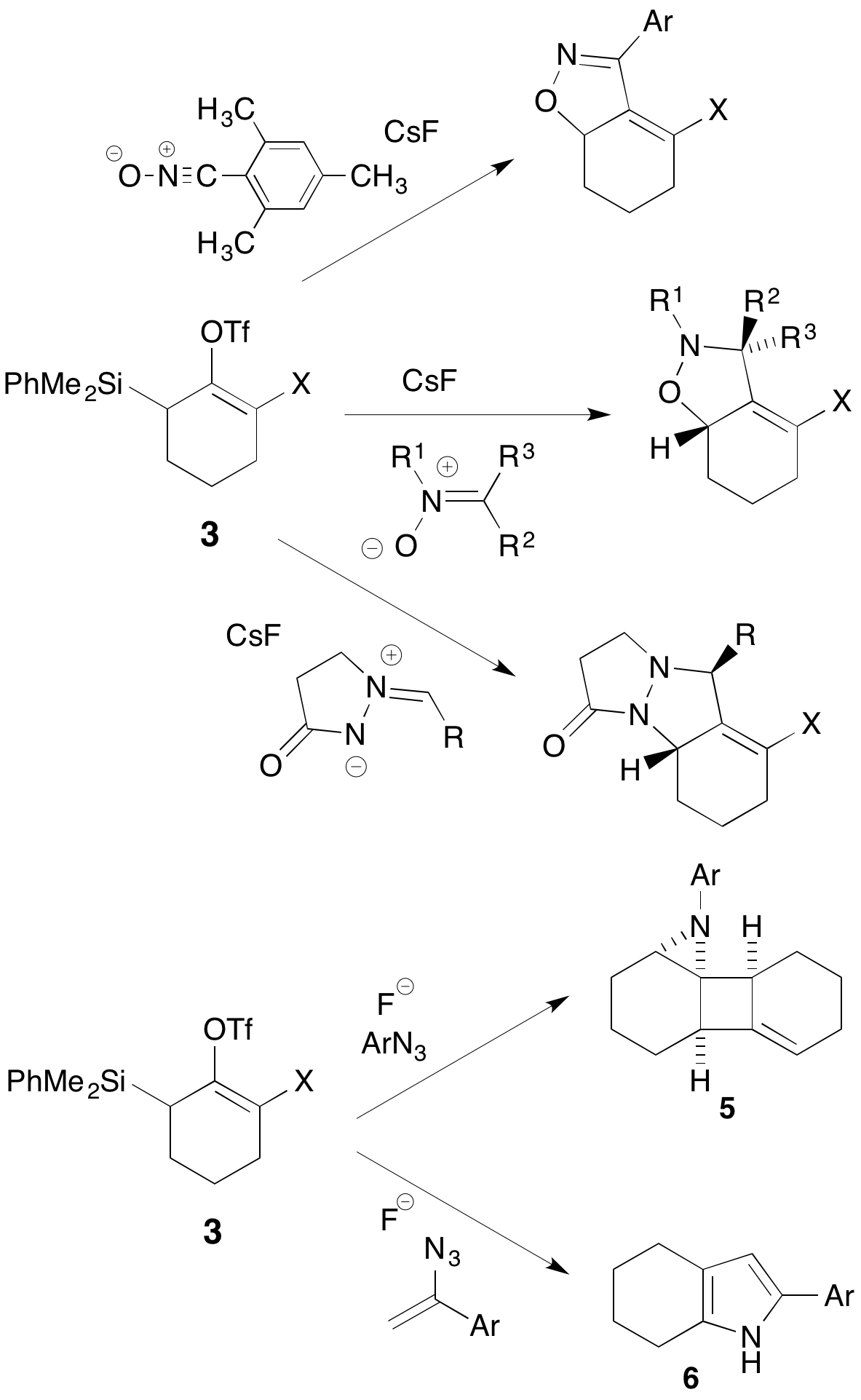
We have found that a wide range of stable 1,3-dipoles can intercept the electron-neutral allenes 4, including nitrile oxide, nitrones, and azomethine imines (Scheme 2). On the other hand, aryl azides form unprecedented 2:1 adducts 5, while styryl azides furnish tetrahydroindoles 6. These novel transformations are under study as potential routes to complex natural products. Electron-deficient allenes 2 show no propensity to react with 1,3-dipoles, but can be trapped with a variety of 1,3-dienes in an efficient Diels-Alder cycloaddition process.
Scheme 2
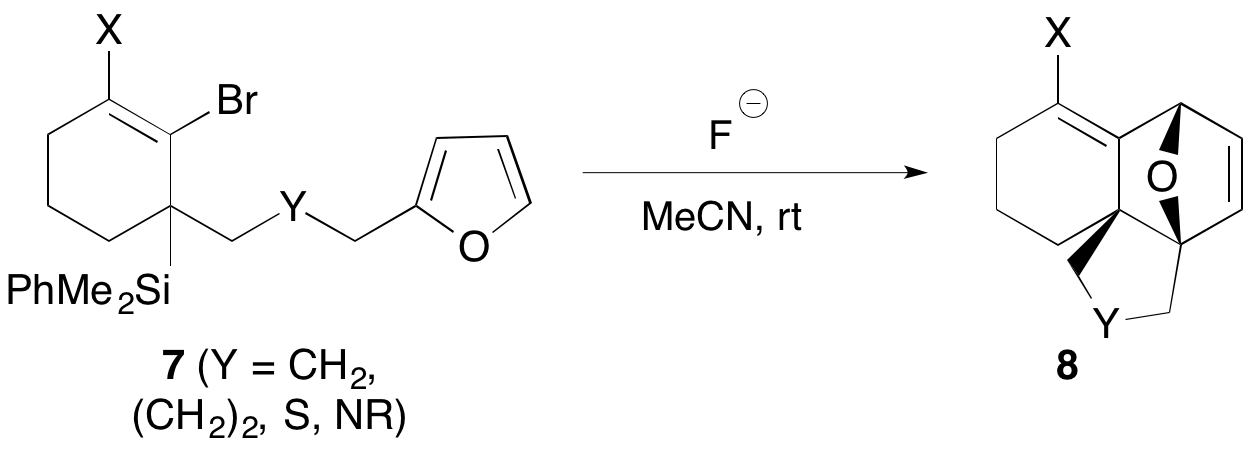
We have also shown that allene precursors such as 7 can undergo highly efficient intramolecular Diels-Alder trapping to afford polycyclic adducts 8 with high or complete diastereoselectivity (Scheme 3). It is notable that no activation (either thermal or Lewis acid) is required for this cycloaddition to occur, offering strong evidence for the enhanced reactivity of the allenes via their high-energy ground states. We are currently exploring other unconventional intramolecular traps to take advantage of this reactivity.
Scheme 3
In the coming year, we will be exploring chirality transfer from optically-enriched allene precursors, as well as exploring alternative methods of allene generation that would permit the use of complementary substrates.