Reports: ND552848-ND5: Interfacial Viscoelasticity of Crude Oils: The Role of Asphaltenes
Gerald G. Fuller, PhD, Stanford University
This research effort applied the methods of interfacial rheology to examine two problems associated with the production of oil: the influence of low salinity salt in the enhancement of oil recovery and the kinetics of hydrate formation at hydrocarbon/water interfaces. Both projects resulted in publications and conference presentations.
Injection of optimized chemistry water in enhanced oil recovery (EOR) has gained much interest in the last few years. Crude oil-water interfaces can have a viscoelastic character affected by the adsorption of amphiphilic molecules. The brine concentration as well as surfactants may strongly affect the fluid-fluid interfacial viscoelasticity. In this work we investigated interfacial viscoelasticity of two different oils (one from the Gulf of Mexico [X], and the other from the Middle East [Y]) in terms of brine concentration and a nonionic surfactant (a nonionic surfactant; the main structure is an ethoxylated resin, provided by CECA, France). We correlated these measurements with oil recovery in a glass-etched flow microchannel.
Interfacial viscoelasticity was found to develop relatively quickly in both oils, and stabilized at about 48 hours. The interfaces were found to be more elastic than viscous. The interfacial elastic (G') and viscous (G") moduli increased as the salt concentration decreased until a maximum in viscoelasticity was observed around 0.01% wt. of salt. Monovalent (Na+) and divalent (Mg2+) cations were used to investigate the effect of ion type; no difference was observed at low salinity. The introduction of a small amount of a surfactant (100 ppm) increased the elasticity of the crude oil–water interface at high salt concentration. These effects are shown in Figure 1 for crude oil X. Two notable results are displayed: the strong enhancement of elasticity at an optimal salt concentration in the absence of surfactant and the diminution of surface viscoelasticity with the addition of the DEM surfactant.
Aqueous solutions that give the maximum interface viscoelasticity, and high salinity brines were used to displace oil in a glass-etched 'porous media' micromodel. Pressure fluctuations after breakthrough were observed in systems with high salt concentration while at low salt concentration there were no appreciable pressure fluctuations. Oil recovery increased by 5-10% in low salinity brines. By using a small amount of a nonionic surfactant with high salinity brine, oil recovery was enhanced 10% with no pressure fluctuations.
Interface elasticity reduced the snap-off of the oil phase leading to reduced pressure fluctuations. This study sheds light on significance of interface viscoelasticity in oil recovery by change in salt concentration and by addition of small amount of a nonionic surfactant.
Our work on hydrates offers a new approach to study and understand the kinetics and mechanical properties of these hydrocarbon/water interfacial structures by interfacial rheology. This is made possible using a "double wall ring" interfacial rheology cell that was been designed to provide the necessary temperature control to explore hydrate phase transitions. Cyclopentane and water were used to form hydrates and this model system forms these structures at ambient pressures.
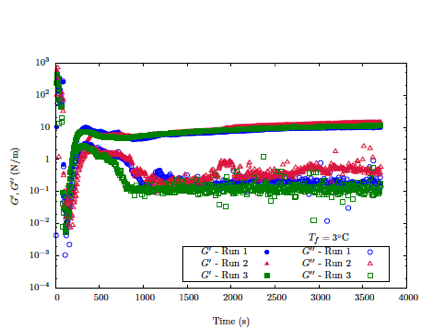
Different temperature and water/hydrocarbon contact protocols were explored. Of particular interest was the importance of first contacting the hydrocarbon against ice crystals in order to initiate hydrate formation. Indeed, this is found to be the case, even though the hydrates may be created at temperatures above the melting point of ice. Once hydrates completely populate the hydrocarbon/water interface, strain and frequency sweeps of the interfacial elastic and viscous moduli were conducted to interrogate the mechanical response and fragility of the hydrate films. The dependence on temperature, Tf , of the kinetics of formation and the mechanical properties was measured and the cyclopentane hydrate dissociation temperature was found to be between 6C and 7C. The formation time (measured from the moment when cyclopentane first contacts ice crystals) as well as the elastic modulus and the yield strain increase as Tf increases. Figure 2 shows the evolution of the elastic and viscous moduli of the cyclopentane/water interface following the step change of temperature from -5C to 3C. Note the slow growth of both moduli over the course of approximately 1000 seconds with the elastic modulus being two orders of magnitude larger than the viscous modulus.