Reports: ND355495-ND3: Homogeneous Nanoparticle Catalysis: Synthesis, Structural Characterization, and Catalysis Applications of Molecular Transition Metal Clusters
Jonathan Owen, PhD, Columbia University
Introduction
To lower the concentration of atmospheric CO2 it is essential to transform CO2 into hydrocarbon fuels and monomers for polyolefin synthesis. En route to this goal we are developing nanoribbon catalyst substrates to which catalysts can be tethered. We are pursuing these structurally well-defined graphene nanoribbon electrodes by the transition metal catalyzed unzipping of carbon nanotubes. Nanoribbon substrates produced in this way can in principle display a density of edge sites that is comparable to metal-organic frameworks or zeolite substrates. With these highly edge dense substrates, we can achieve unprecedented levels of selectivity, activity, as well as improved structural insights into the relationship between catalyst structure and function. By tuning the ligand environment of the covalently attached molecular catalyst and the nature of the attachment to the graphene electrode material, we aim to understand the structure-function relationships that control the electrocatalytic reduction of CO2 to hydrocarbon fuels and olefins such as ethylene and propylene.
Catalytic unzipping of carbon nanotubes
Our first efforts have focused on optimizing the transition metal catalyzed unzipping of single walled carbon nanotubes to generate structurally well-defined graphene nanoribbons. Various literature methods have shown the viability of using the inherent strain energy in carbon nanotubes to drive C-C bond cleavage down the longitudinal axis of the nanotubes.1–3 These methods often result in nanoribbons with diminished electrical properties (due to defects in the basal plane) or ambiguous edge functionalities. For example, graphene oxide nanoribbons made from the oxidation of CNTs using KMnO4 result in a combination of carbonyls, ethers, and carboxyls,4 making a precise understanding of the influence of edge functional group on catalytic activity difficult.
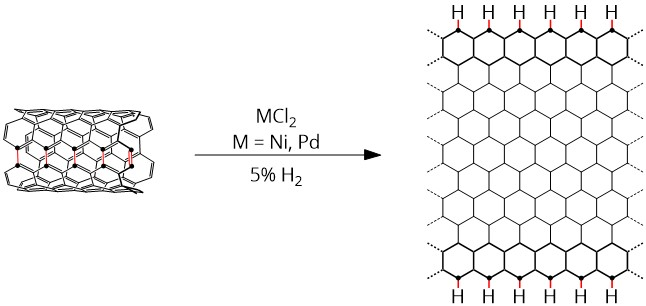
Our strategy to synthesize a well-defined nanoribbon product is to use transition metals to activate the C-C bonds of the nanotube in an atmosphere of reactive gasses. Instead of using highly oxidizing reagents that can damage the conjugated basal carbons, more mild conditions will preserve the electronic character of the graphene. Commercially sourced single walled carbon nanotubes (SWCNT) were treated with metal chloride salts (e.g. NiCl2, PdCl2á(CH3CN2), RuCl3) and exposed to 5% H2 atmosphere, shown in Scheme 1, in a diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) cell.
Scheme 1: longitudinal unzipping of a single walled carbon nanotube using group 10 metal chlorides in hydrogen atmosphere
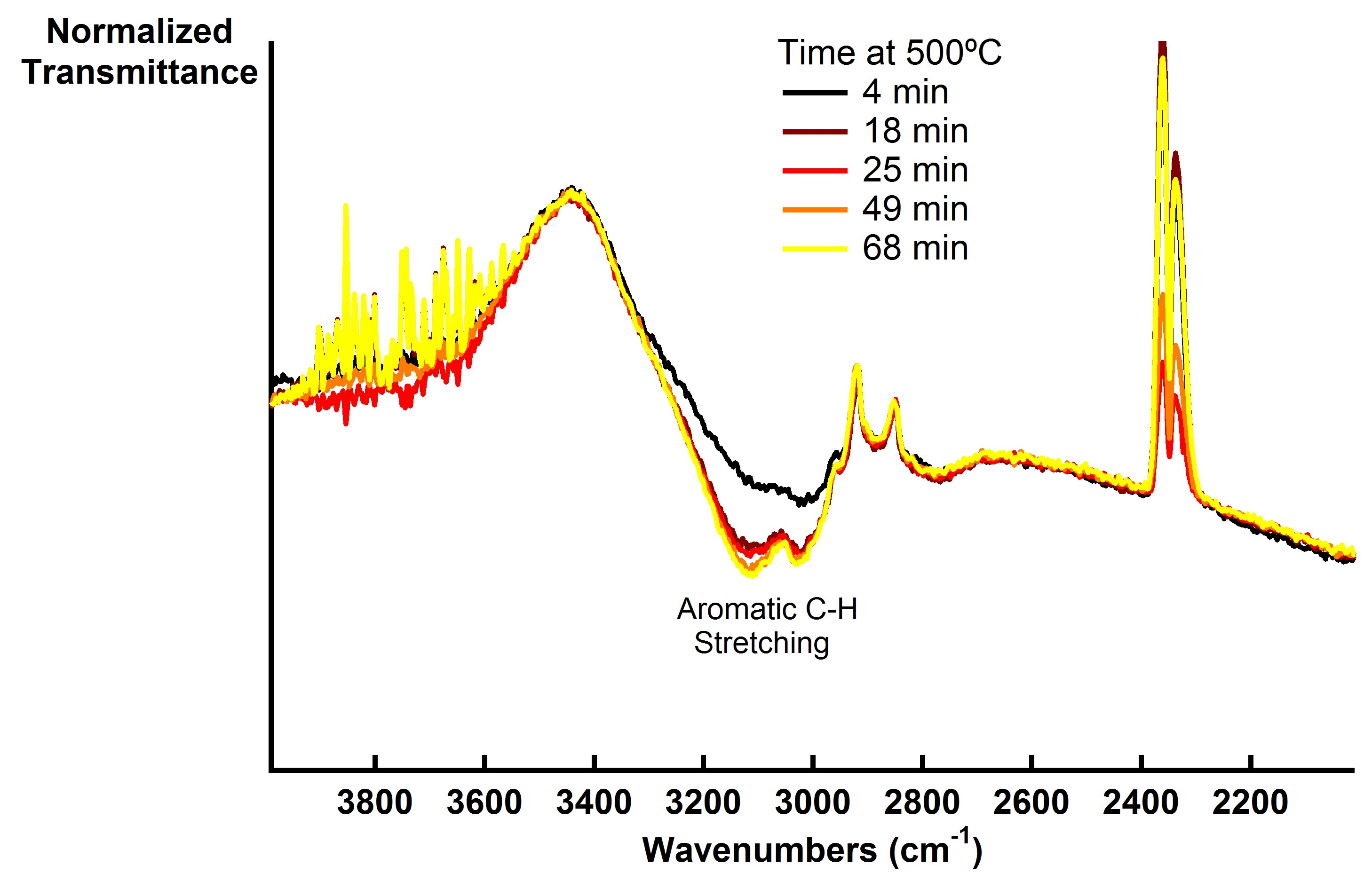
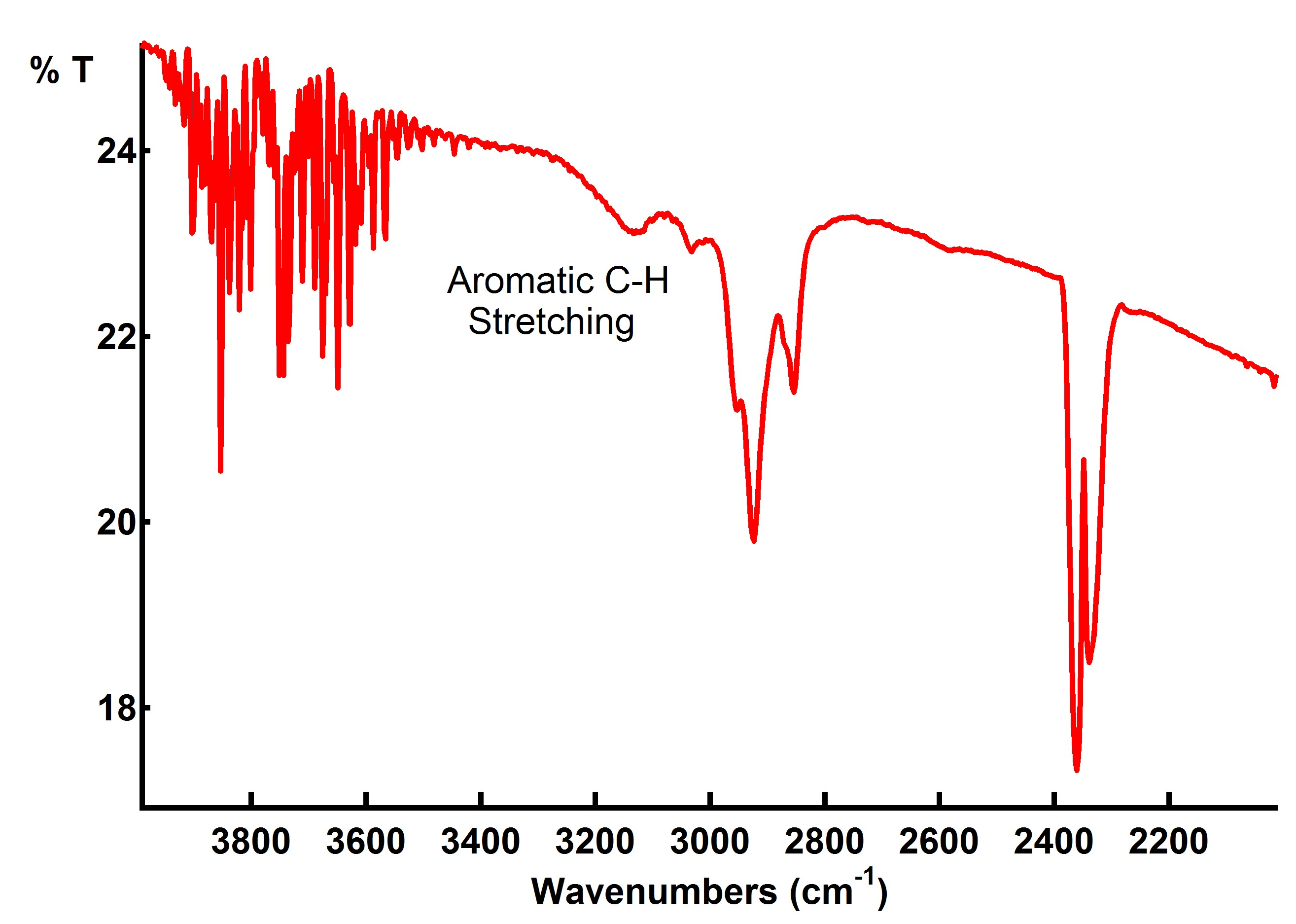
Preliminary results with nickel (Figure 1) and palladium (Figure 2) suggest that unzipping and hydrogenation can be achieved under 5% hydrogen at 500°C similar to previous results using multi-walled CNTs.2 As the reaction progresses, signal associated with aromatic C-H stretching at 3030 and 3120 cm-1 indicates unzipping and the incorporation of hydrogen. The broad signal at ~3400 cm-1 is an O-H signal from residual EtOH used in sample preparation that was driven off under the reaction conditions, atmospheric CO2 also appears from 2300-2400 cm-1, and significant scattering from the carbon nanotubes contributes to a strong background signal. We are currently studying the structure of the hydrogenated product using Raman spectroscopy and electron microscopy.
Figure 1: in-situ DRIFTS spectra of hydrogenation of graphene nanoribbons from NiCl2 decorated SWCNTs in 5% H2 atmosphere
Figure 2: in-situ DRIFTS spectra of hydrogenation of graphene nanoribbons from PdCl2á(CH3CN)2 decorated SWCNTs in 5% H2 atmosphere
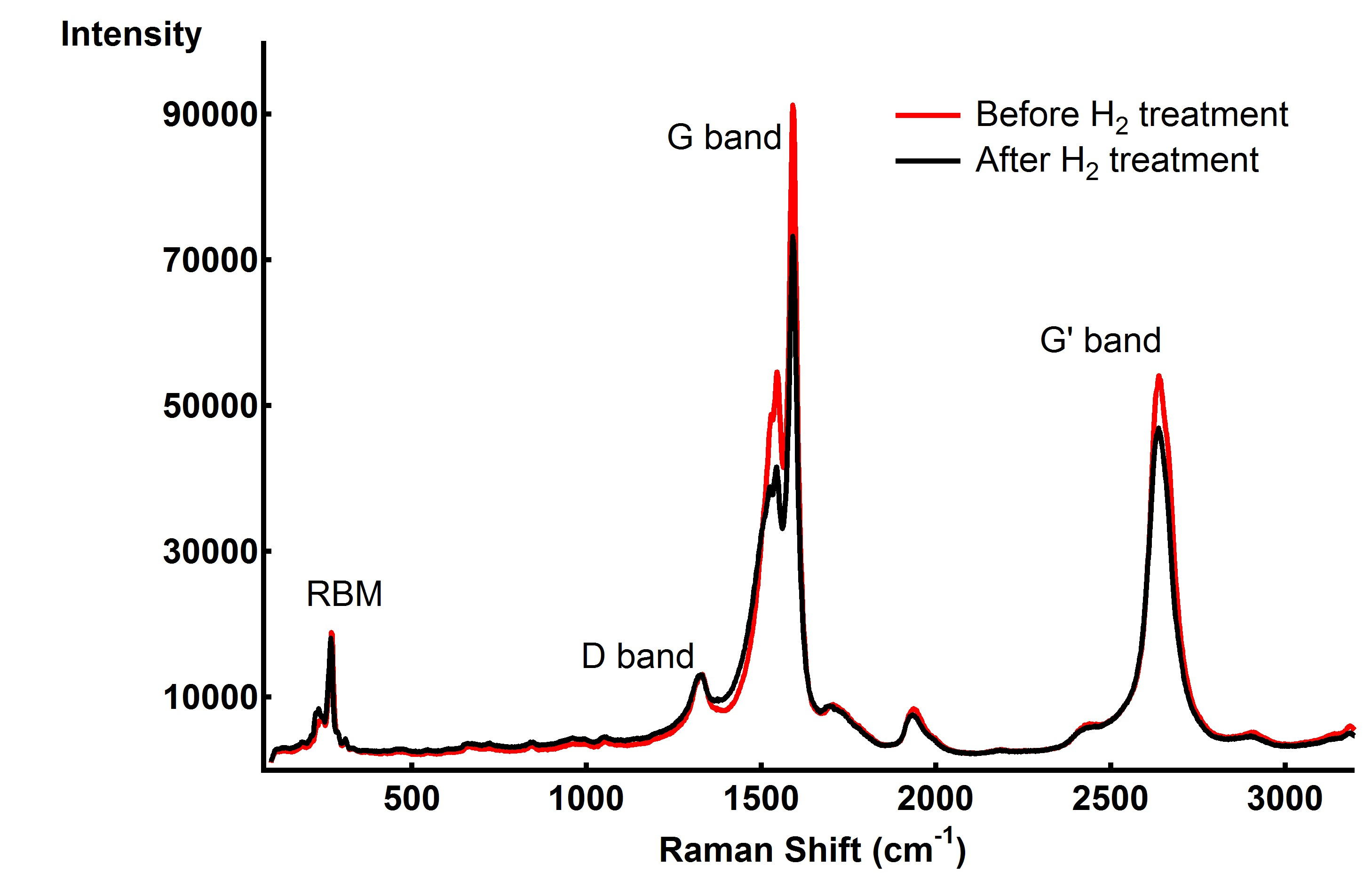
Raman spectroscopy of SWCNTs before and after metal/hydrogen treatment (Figure 3) shows minor changes, consistent with the incomplete hydrogenation. A shift of 2 cm-1 in the radial breathing mode (RBM) and an increase ratio of the D/G band (an indication of increased defect or edge states) do point to successful unzipping, however the bundling together of SWCNTs can also cause some spectral changes.
Figure 3: Raman spectra of SWCNT before and after H2 treatment at 500°C. Although changes are present bundling of nanotubes convolutes data analysis.
Controlling the functionalization of graphene nanoribbons
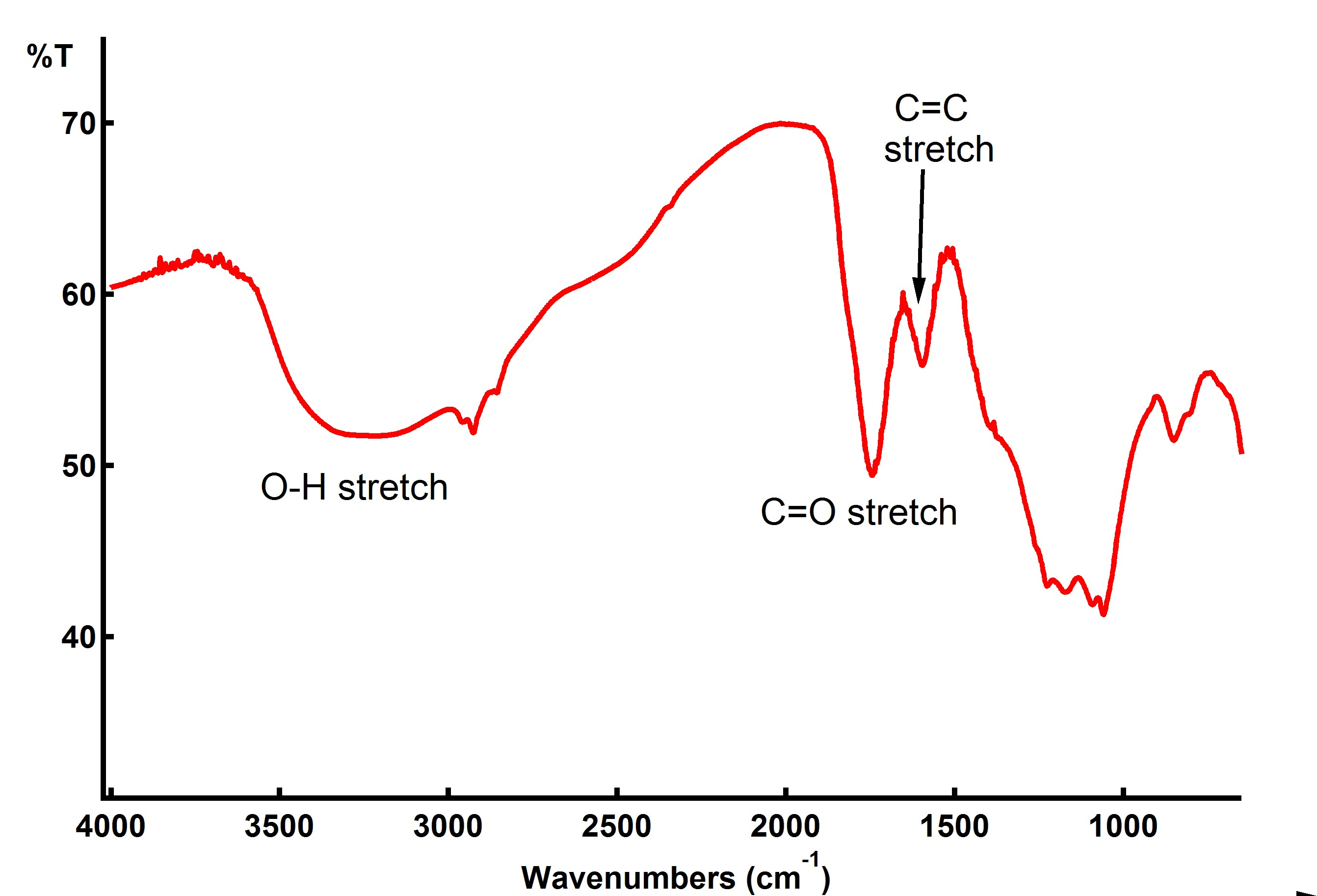
Using an adapted synthesis from Acik et al.4 we were able to unzip SWCNTS and add oxygen functional groups to the nanoribbon edges. The high weight percent of edge carbons in thin nanoribbons allows stronger signal in the FT-IR spectra (figure 3).
Figure 4: DRIFTS spectra of graphene oxide nanoribbons prepared from KMnO4 and H2SO4 solution. Carbonyl and carboxyl functional groups are present.
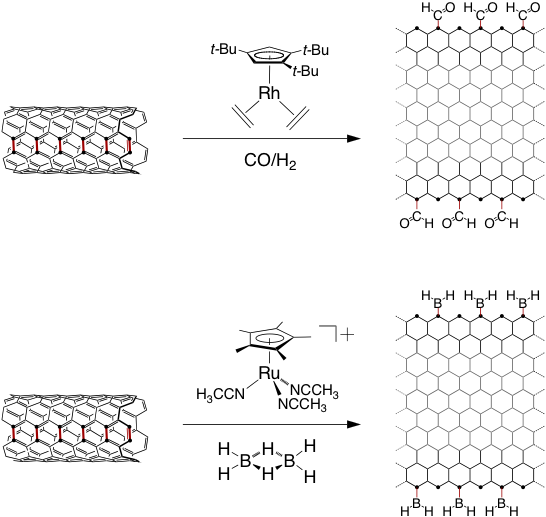
Creating molecular linkages to the graphene nanoribbon electrodes with high fidelity and selectivity will require precise control of the edge structure. Transition metal complexes with high thermal stability have been chosen for their ability to catalytically cleave the C–C bonds of cyclopropane substrates. Performing the transition metal catalyzed solid state reactions described above in the presence of other reactive gases, such as borane, silane, and carbon monoxide, will allow us to prepare nanoribbons with a wide variety of functionalities (Scheme 2).
Scheme 2: Longitudinal unzipping of a SWCNT via hydroformylation and hydroborylation using metal-arene catalysts.
Attachment of molecular catalyst to graphene nanoribbon electrode
After protocols are developed for the precise control and characterization of graphene nanoribbon electrodes is complete our attention will turn to catalyst design and study. Tuning the metal identity and ligand shell will allow for selectivity of carbon based products and the efficiency of the catalytic processes. In addition, the attachment of a molecular catalyst to a well-defined, functionalized electrode allows us to probe the relationship between the structure of the transition metal site and its activity. We look to explore how changing the linkage between the molecular catalyst and the electrode can affect the efficiency, selectivity, and products of CO2 reduction with a high degree of structural clarity.
References:
(1) Kosynkin, D. V.; Higginbotham, A. L.; Sinitskii, A.; Lomeda, J. R.; Dimiev, A.; Price, B. K.; Tour, J. M. Nature 2009, 458 (7240), 872–876.
(2) El’as, A. L.; Botello-MŽndez, A. R.; Meneses-Rodr’guez, D.; Jehov‡ Gonz‡lez, V.; Ram’rez-Gonz‡lez, D.; Ci, L.; Mu–oz-Sandoval, E.; Ajayan, P. M.; Terrones, H.; Terrones, M. Nano Lett. 2010, 10 (2), 366–372.
(3) Wang, J.; Ma, L.; Yuan, Q.; Zhu, L.; Ding, F. Angew. Chem. Int. Ed. 2011, 50 (35), 8041–8045.
(4) Acik, M.; Carretero-Gonz‡lez, J.; Castillo-Mart’nez, E.; Rogers, D. M.; Guzman, R.; Baughman, R. H.; Chabal, Y. J. J. Phys. Chem. C 2012, 116 (45), 24006–24015.