Reports: ND655842-ND6: Understanding the Photoluminescence Mechanism of Water Soluble Polymer Clusters for Petroleum Exploration
Liangfeng Sun, PhD, Bowling Green State University
Overview
The goal of the proposed research is to find out the light-emitting mechanism of water soluble polymer clusters for petroleum exploration. In the past year, we have developed two important experimental apparatus to measure the absolute quantum yield and photoluminescence lifetime of the material, besides the general optical spectroscopy and electron microscopy characterizations. Based on the preliminary data, we conclude the light-emitting from the polymer clusters is unlikely due to the radiative relaxation of a triplet state as reported for polyamides in the literature, since its radiative lifetime is short (~ns) even at temperature as low as 32 K. The absolute quantum yield of the polymer clusters peaks at around 450 nm, showing a molecular signature. These results have been summarized in an abstract submitted to 2017 MRS Spring Meetings & Exhibit.
Result 1: Optical Absorption and Photoluminescence
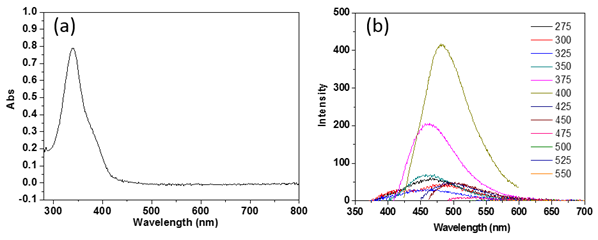
As shown in Figure 1, the optical absorption mainly occurs in the UV range of the spectrum while the photoluminescence occurs in the visible range. This difference indicates that the emitting states might be different from absorption states. It is also known that the photoluminescence peak depends on the excitation wavelength (Figure 1b) when the excitation wavelength is equal or longer than 400 nm, but does not change much if the excitation wavelength is less than 400 nm. This strongly indicates that the polymer clusters have two different types of photoluminescence states: molecule-like states and surface states. The photon emission of molecule-like states follows Kasha’s rule [1], i.e., the emission wavelength is independent of the excitation wavelength. Its photoluminescence peaks at around 460 nm. The emission of surface states is typically excitation-wavelength dependent [2-4]. This is an intriguing result since it is now possible to selectively excite either type of states for a further study of the radiative relaxation lifetime using time-resolved photoluminescence.
Figure 1. (a) Absorption spectrum of polymer clusters dispersed in water. (b) Photoluminescence from polymer clusters dispersed in water, excited at different wavelength.
Results 2: Absolute Photoluminescence Quantum Yield
The apparatus to measure the absolute quantum yield includes a wavelength tunable laser, an integrating sphere, a spectrometer and a silicon detector.[5] A few “standard” chromophores have been measured and their absolute quantum yields are compared with the data from the literature. The difference is circa a few percent.
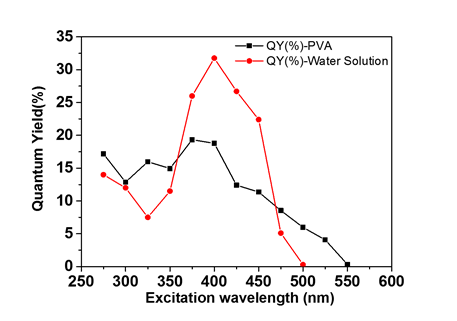
This method has been used to measure the absolute quantum yield of the polymer clusters. Two samples were prepared: one is the polymer clusters dispersed in Poly(vinyl alcohol) (PVA), the other is the same clusters dispersed in water. For both samples, the photoluminescence quantum yield depends on the excitation wavelength. Interestingly, the photoluminescence quantum yield reaches maximum when the excitation wavelength is between 375 nm to 400 nm, while the maximum absorption occurs at 340 nm (Figure 1a). Comparing with results shown in Figure 1, we may draw a conclusion that the highest quantum yield comes from the emission of the molecule-like states.
Figure 2. Photoluminescence quantum yields (QYs) of the polymer clusters dispersed in PVA (black squares) and in water (red dots).
Results 3: Photoluminescence Lifetime
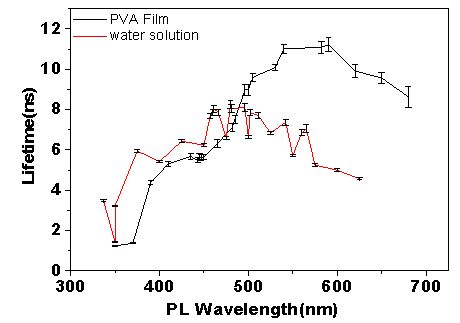
Time-resolved photoluminescence is measured by a fast optical detector while using a laser pulse to excite the sample at t=0. In our experiment, the polymer clusters were excited by a pulse laser (wavelength 337 nm, pulse duration 6 ns). Two samples (clusters in PVA film and clusters in water) were prepared for the measurements. The photoluminescence lifetime at different wavelength from each sample was measured. As show in Figure 3, both samples show increased lifetime as the wavelength increases. This is consistent with the results show in Figure 1, the emission at wavelength longer than 400 nm is likely form surface states.
Figure 3. Photoluminescence lifetime of the polymer clusters dispersed in PVA (black line) and in water (red line) at room temperature.
Results 4: Temperature Effect on the Photoluminescence Lifetime
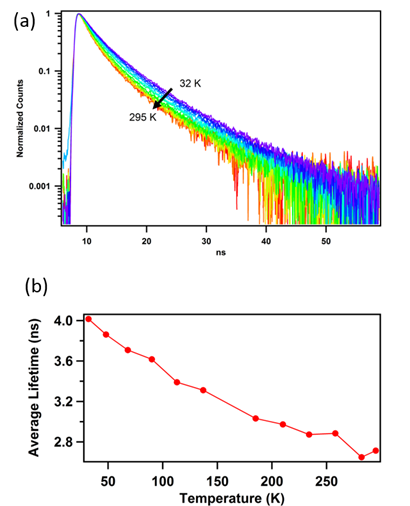
The photoluminescence lifetime is affected by the temperature. Particularly, the radiative decay of the excited triplet states are strongly competed by the non-radiative relaxation at room temperature, which makes it difficult to obtain the triplet information. To reduce the non-radiative rate, the lifetime measurements were performed at cryogenic temperatures. As shown in Figure 4, the lifetime of the polymer clusters dispersed in PVA was measured at different temperature. We observed continuous increase of the lifetime as the temperature decreases. This is probably due to the less effect of phonons at low temperatures. However, the lifetime is still short, about 4 ns at 32K. It is unlikely the emission is from triplets.
Figure 4. Temperature dependent photoluminescence lifetimes. The sample is excited by 400 nm laser pulses. The lifetime was measured at 500 nm.
References
[1] M. Kasha, Discuss. Faraday Soc. 1950, 9, 14.
[2] Y. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca, S. Xie, J. Am. Chem. Soc. 2006, 128, 7756.
[3] Z. Ding, B. M. Quinn, S. K. Haram, L. E. Pell, B. A. Korgel, A. J. Bard, Science 2002, 296, 1293.
[4] J. D. Holmes, K. J. Ziegler, R. C. Doty, L. E. Pell, K. P. Johnston, B. A. Korgel, J. Am. Chem. Soc. 2001, 123, 3743.
[5] J. C. de Mello, H. F. Wittmann, R. H. Friend, Adv Mater 1997, 9, 230.