Reports: DNI554217-DNI5: In Situ Characterization of Small Organic Molecule Oxidation Using Stimulated Raman Spectroscopy
Jin Suntivich, Cornell University
This project seeks to understand the surface chemistry of a catalyst during a small molecule oxidation reaction. One of the largest hurdles in the catalyst design is the lack of information about the surface chemistry of a catalyst. Common in situ electronic probes, such as X-ray spectroscopy, do not directly reveal structural information, while vibrational probes such as infrared spectroscopy do not efficiently operate in aqueous condition. Raman spectroscopy is one of the few vibrational probes that can operate well in water. However, the signal generated is often inadequate for surface studies.
This project seeks to overcome this signal limitation by harnessing stimulated Raman spectroscopy (SRS). SRS relies on the stimulated emission concept to increase efficiency1. This is in contrast to surface-enhancement methods such as surface-enhanced infrared absorption spectroscopy (SEIRAS) and surface-enhanced Raman spectroscopy (SERS), which rely on the evanescent field. Unlike SERS/SEIRAS, which may not resolve the bulk average information due to its extreme sensitivity to the high-electric-field regions, SRS can probe the catalyst’s surface homogeneously due to its free-space nature.
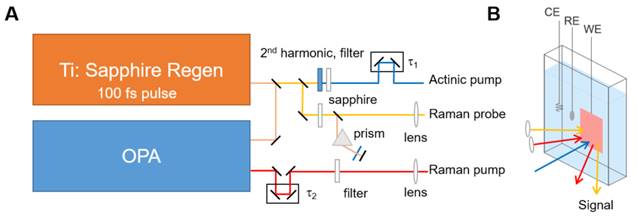
Driven by the possibility that in situ SRS may serve as a new tool for examining the surface of a catalyst during an oxidation reaction, we construct an in situ electrochemical cell integrated with SRS with the support of the ACS PRF. Our design is shown in Figure 1A-B. On the optical side, we use two optical beams to carry out SRS. The pump beam is produced from the optical parametric amplifier (OPA) and is spectrally narrowed. The probe beam is produced from a white-light continuum generation from the sapphire plate. We use the optical Kerr effect to find the equal path lengths between the beams.
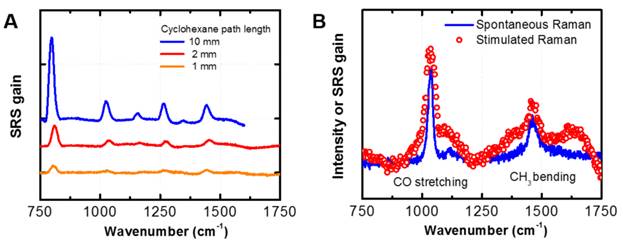
Over the past year, we have increased the reliability of our SRS probe. Figure 2A show example SRS spectra of cyclohexane, using a pump wavelength of 633 nm as a function of the path length of a cell containing cyclohexane, collected in our laboratory. We show that our SRS experiment can resolve a sample with a beam path of 1 mm. Figure 2B show an example SRS spectra for methanol, demonstrating the similarity between spontaneous Raman and SRS. We note that we have not yet optimized the signal-to-noise ratio for the methanol SRS spectrum. We anticipate better results in the future.
Our next step in the extended period is to integrate the SRS experiment and in situ electrochemical cell that we had developed in the first year of the support period. Notably, we modify the design from the Berkeley and Leiden groups2,3, which used an upward/downward-facing design to sided window (Figure 1B). With the incorporation of SRS-electrochemical cell, we will examine the methanol oxidation reaction and test how the methanol is oxidized (to carbon monoxide, then carbon dioxide.) Finally, an actinic pump will be integrated (Figure 1A) to trigger the reaction, allowing a full experimentation on not only the surface chemistry but also the time evolution of it.
The support from the ACS-PRF has had an immense impact on my career and the students who participated in the project. On my career front, this project enables us to pursue a high-risk, high-reward project that integrates two normally distinct disciplines: ultrafast spectroscopy and electrochemistry. Through this pursuit, we will be able to test the hypothesis of whether advanced spectroscopy can be utilized to find new catalysts. The impact on the education of the students in my group is also significant. This program has supported two very bright Cornell Ph.D. students to learn cutting-edged concepts and skills in ultrafast spectroscopy, surface science, and electrochemistry. The graduate students supported had taken on a mentorship role of a younger undergraduate student, who is sponsored by the Cornell undergraduate research fund. Together, they have learned how to work in a collaborative environment and the analytical skills necessary to succeed in research.
1. McCamant, D. W., Kukura, P., Yoon, S. & Mathies, R. A. Femtosecond broadband stimulated Raman spectroscopy: Apparatus and methods. Rev. Sci. Instrum. 75, 4971–4980 (2004).
2. Yeo, B. S. & Bell, A. T. In situ raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 116, 8394–8400 (2012).
3. Diaz-Morales, O., Calle-Vallejo, F., de Munck, C. & Koper, M. T. M. Electrochemical water splitting by gold: evidence for an oxide decomposition mechanism. Chem. Sci. 4, 2334–2343 (2013).
Figure 1. (A) Schematic drawing of the SRS experiment. A Ti:sapphire regenerative amplifier’s fun-damental is split into three beams. The three beams are (i) Raman pump, which is created using the optical parametric amplifier (OPA) to shift to the 633 nm wavelength (in order to keep ~3,000 cm–1 within the Si detector bandwidth) and then spectrally narrowed, (ii) Raman probe, which is created from a continuum generation, and (iii) actinic pump, which is created via the 2nd harmonic generation in a barium borate crystal. (B) Schematic drawing of the used in situ electrochemical cell for the SRS experiment. All three beams are incident on the surface during the methanol oxidation reaction. CE: Counter electrode; RE: Reference electrode, WE: Working electrode.
Figure 2. (A) Exemplary SRS spectra on cyclohexane at 633 nm pump wavelength collected in our laboratory. The signal linearly decreases with the cyclohexane path length. Our current setup allows us to conduct SRS within < 1 mm of liquid cell thickness. (B) Our preliminary Raman spectra of methanol, showing the similarity between SRS and spontaneous Raman spectra.