Reports: UNI354044-UNI3: Preparation, Electronic Structure, and Reactivity Studies of Iron Complexes Supported by Conjugated Alpha-Diimine Ligands
Helen Hoyt, PhD, Knox College
Overview: Progress in the second grant year has continued to focus on the synthesis, characterization, electronic structure, and catalyst activity of iron(II) bromide complexes supported by conjugated a-diimine ligands. We have investigated two related ligand classes: (a) the bidentate aryl bis(imino)acenaphthene (Ar-BIAN) ligands and the aryl a-diimine (Ar-DI) ligands, along with (b) the tridentate pendant donor-modified aryl bis(imino)acenapthene ligands. Building upon our results from the first grant year, continued progress toward elucidating the structural factors contributing to an effective iron catalyst for the functionalization of olefins is described below.
A. Synthesis, electronic structure, and catalytic hydrosilylation studies of iron complexes supported by bidentate ligands
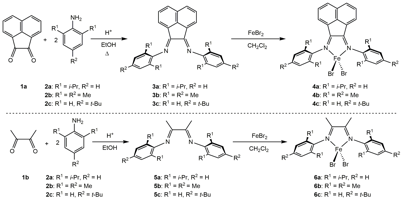
Anhydrous iron dibromide complexes (4a-c and 6a-c, Figure 1) bearing the bidentate a-diimine Ar-BIAN (3a-c) and Ar-DI (5a-c) ligands (Ar represents: (a) dpp = 2,6-diisopropylphenyl; (b) Mes = 2,4,6-trimethylphenyl; (c) 4tBu = para-tert-butylphenyl; and BIAN = bis(imino)acenaphthene) were prepared in two steps from commercially available starting materials (1a-b and 2a-c). Characterization methods for ligands (e.g., 3c, 5c) and iron dibromide complexes generally included Nuclear Magnetic Resonance (NMR) spectroscopy, Infrared spectroscopy (IR), and X-ray diffraction (3c, 4a, 4b, 5c, 6c). Purity was assessed by melting point and elemental analysis.
Figure 1. Synthesis of (Ar-BIAN)FeBr2 (4a-c) and (Ar-DI)FeBr2 (6a-c).
Electronic structure studies of iron complexes 4a and 4b were conducted primarily by Mössbauer Spectroscopy to assess the spin state of the iron metal center, along with magnetic measurements by the Evans' (NMR) method. Quantum chemical density functional theory computations were performed at the B3LYP level to provide insights into the electronic structure of compounds 4a and 4b; the computational results were found to be in acceptable agreement with the experimental metrical and Mössbauer parameters. Additionally, numerical frequency computations were performed at the same level of theory to confirm that a global energy minimum had been located and that there were no imaginary frequencies for both complexes. Qualitative molecular orbitals and spin density plots were generated to describe the high spin Fe(II) electronic structure of the metal centers supported by a redox-innocent Ar-BIAN chelate for both 4a and 4b, providing a baseline for comparison of Ar-BIANFe complexes bearing redox-active chelates.
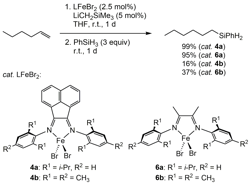
Preliminary experiments indicated that in situ reduction of all precatalysts, 4a-c and 6a-c, promoted the catalytic hydrosilylation of hydrocarbons. More specifically, upon in situ reduction with LiCH2SiMe3, the iron complexes 4ab and 6ab promoted catalytic hydrosilylation of 1-hexene with phenylsilane at ambient temperature, with highest yields provided by precatalyst dppBIANFeBr2 (4a) followed closely by parent a-diimine precatalyst dppDIFeBr2 (6a, Figure 2). The smaller N-substituents for 4b and 6b may have allowed for the formation of less active iron complexes with a higher coordination number. Under optimized conditions, 1 mol% 4a was activated in situ with 2 mol% LiCHSiMe3 to produce 1-hexylphenylsilane in 95% yield from a 1:1 ratio of 1-hexene:PhSiH3 substrates under solvent-free conditions at 22 oC in 24 hours. Our current focus is on optimizing catalysis for 4c and 6c to elucidate the effect of para-substitution on this hydrosilylation reaction.
Figure 2. Catalytic hydrosilylation of 1-hexene with phenylsilane upon in situ activation of precatalysts 4a/b and 6a/b with LiCH2SiMe3.
B. Electronic structure studies of iron dibromide complexes supported by conjugated tridentate ligands
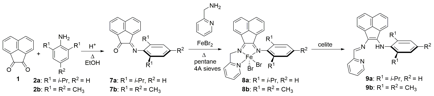
Building upon the work from the first grant year, the synthesis of two pendant donor N-modified α-diimine bis(imino)acenaphthene (BIAN) ligands (dppNNNH or 9a and MesNNNH or 9b), as well as iron dibromide complexes supported by a tautomer of these ligands (dppNNNFeBr2 or 8a and MesNNNFeBr2 or 8b) is described in Figure 3. The pendant donor is a pyridine ring connected to the conjugated BIAN ligand through a single-carbon bridge to make tridentate ligands. The free ligands were successfully isolated as tautomers (NNNH) of the bound ligands (NNN). Characterization methods included NMR and IR spectroscopy, melting point, elemental analysis, X-ray diffraction, Mössbauer Spectroscopy, and magnetic measurements by the Evans' (NMR) method, as needed. Of particular focus in this second grant year were magnetic Mössbauer measurements obtained under a range of magnetic fields for compound 8a, and the electronic structure description as a high spin iron(II) metal center bound by a redox-innocent dppNNN ligand has additionally been investigated and supported by density functional theory computations. Preliminary experiments indicate that in situ reduction of complex 8a promoted the hydrosilylation of hydrocarbons with phenylsilane at ambient temperature in modest yield; our current focus is to optimize the conditions for this process.
Figure 3. Synthesis of NNNFeBr2 complexes 8a/b and tautomeric NNNH ligands 9a/b.
Impact: Two undergraduate research students were supported in the second grant year (9/2015-8/2016). This experience has afforded the students an opportunity to learn important experimental techniques and skills in inorganic chemistry, including the synthesis and handling of air-sensitive compounds, interdisciplinary characterization techniques with the physics department at Knox College and the crystallography research center at Eastern Illinois University, and communicating their research results through written reports and research presentations. These experiences have been extremely beneficial for these two current students (classes '17 & '18, respectively) as they plan to apply to graduate programs in chemistry. One of the students supported in the first grant year graduated in 2016 and is currently enrolled in the PhD program in chemistry at Iowa State University, while the other 2016 graduate plans to apply to medical school.
The results of this project have so far been disseminated through a journal article published in Polyhedron and presentations at meetings. The students have presented at the Midstates Consortium for Mathematics and Science 2015 Undergraduate Research Symposium at The University of Chicago, and the 251st National Meeting of the American Chemical Society in San Diego, CA. The PI also presented at the 251st National Meeting of the American Chemical Society in San Diego, CA.