Reports: DNI1055033-DNI10: Discovery of Active Sites on Nanostructured Oxides for Desulfurization
Prashant Jain, PhD, University of Illinois at Urbana-Champaign
Summary and Impact: Our studies are aimed at an atomistic level imaging of chemical transformations of inorganic nanostructures. In particular, our focus has been on the electron microscopy characterization of ion exchange transformations such as:
a) the reaction of nanostructured ZnO with S2- to form ZnS, a reaction with utility in reactive sorption of organosulfur.
b) the cation exchange of CdSe nanostructures to form chalcogenide solids with previously unknown crystallographic structures and novel properties.
While a mechanistic understanding of sulfidation ZnO via in situ studies is currently underway, our recent investigations funded by the DNI grant have taken my group in an exciting new direction. Specifically, we have found unique super-ionic behavior in ultrasmall clusters of the semiconductor Cu2Se synthesized by the chemical transformation of CdSe clusters. These findings reported below have been submitted to the journal Nature Communications.
Background: Cu2Se is a solid with a peculiar ionic structure: the smaller Cu+ ions (eight or fewer per unit cell) have access to a much greater number of crystallographic sites within a rigid cage formed by the significantly larger Se2- anions. The large number of vacant sites available for Cu+ hopping is a primary factor in the manifestation of super-ionic transport in this solid. However, in its low temperature (LT) β phase, the vacancies are ordered and Cu+ ions are localized at the lowest-energy interstitial sites within a lower symmetry pseudo-cubic Se2- sub-lattice. Ionic transport in this form is rather limited. Above ca. 400 K, there exists a high temperature (HT) α phase of Cu2Se, in which the Cu+ ions form a disordered, “liquid-like” sub-lattice. The Cu+ ions are freely mobile between vacant and occupied sites within the immobile, face-centered cubic (fcc) Se2- sub-lattice. This mobile Cu+ network supports Cu+ diffusivities (10-5-10-4 cm2 s-1) as high as those of liquids or molten salts and resulting ionic conductivities of 1-2 Ω-1 cm-1 (at 670 K), three orders of magnitude larger than the room temperature value. This super-ionic behavior is promising for development of solid-state electrolytes. However, the need for high temperatures can be limiting.
Methods: Ultrasmall Cu2Se clusters were prepared by solution-phase cation exchange of magic-sized ca. 2 nm CdSe clusters with Cu+. Due to the topotactic nature of cation exchange, the anionic framework is preserved in the cation exchange process, yielding ultrasmall Cu2Se clusters of similar ca. 2 nm size and fcc Se sub-lattice arrangement as the initial zinc blende CdSe template. For studying the comparative effect of crystallite size on room-temperature ionic structure, larger NCs were also prepared from size-controlled CdSe NCs using the same cation exchange method. The room-temperature crystal structure of the NCs was studied using high-resolution electron microscopy (HRTEM) and X-ray diffraction (XRD).
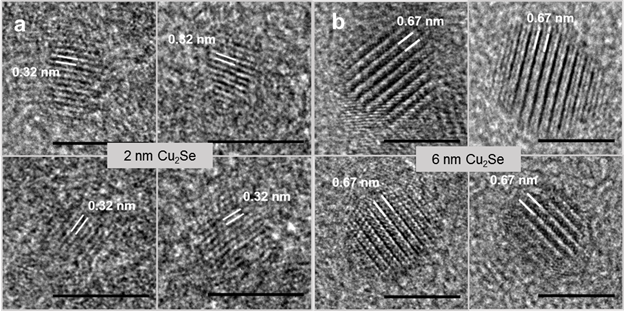
Results: Vacancy ordering below the order-disorder transition (ca. 400 K) temperature is a well-established hallmark of bulk Cu2Se, which we studied in the clusters and the larger NCs using HRTEM. The LT β non-superionic phase has a defective anti-fluorite structure, wherein a fraction of the tetrahedral sites are vacant and the displaced Cu+ ions instead occupy trigonal sites. In the non-super-ionic phase these tetrahedral vacancies stack every four Cu+ layers along the <111> crystallographic axis creating a super-structure with a periodicity of 6.7 Å. Cu+ vacancy ordering is manifested in the form of a lattice fringe contrast pattern in HRTEM with an abnormally large 6.7 Å periodicity. In the HT super-ionic α-Cu2Se phase, the Cu+ ions are mobile between filled and vacant interstitial sites and the super- structural ordering is lost. In this form, regular lattice fringes corresponding to an inter-Se planar spacing of 3.3 Å are seen along the <111> direction.
In analogy to such observations on bulk Cu2Se, we employed HRTEM imaging to characterize at ambient temperature the presence of tetrahedral vacancy ordering and the resulting super-structure in the Cu2Se clusters and the NCs. Several NCs with high-resolution lattice patterns along <111> were analyzed. Representative images of four NCs at each size are shown in the figure above, where the scale bar is 5 nm. The 6 nm NCs displayed a contrast pattern along {111} with a distance of 6.7 Å between bright fringes that represent Cu vacancy planes. This contrast pattern arising from vacancy-ordered super-structure was observed in 80% of the 6 nm NCs, suggesting the existence of these NCs in the non-superionic phase. On the other hand, the 2 nm clusters displayed a complete lack of superstructure, quite unlike 6 nm NCs or bulk Cu2Se at ambient temperature. Lattice fringes along <111> with a 3.2 Å periodicity of adjacent Se planes were seen, characteristic of the super-ionic phase with a mobile, disordered Cu+ sub-lattice. Interestingly, at the intermediate size of 4 nm, super-structural ordering was observed in only 10% of the NCs. Thus, as the crystallite size is reduced from 6 nm to 2 nm, HRTEM shows an increase in the statistical prevalence of a disordered Cu+ sub-lattice with no super-structural ordering.
Conclusions: Thus, ultrasmall Cu2Se clusters exhibit a mobile, “liquid-like” Cu+ sub-lattice at room temperature, whereas such a super-ionic phase is seen in the bulk at significantly higher temperatures. Further investigations by XRD and phonon spectroscopy show that the mobile Cu+ network is linked to a unique cationic sub-lattice structure in the clusters that is remarkably different not just from the bulk form of the solid but also from larger NCs due to the effect of high compressive strain in the clusters. NC size tuning of phase transition temperatures is well known, but the influence of NC size and associated strain on ionic structure and transport is an open area of investigation.