Reports: DNI354504-DNI3: Electron-Donating Carboranyl Ligands for Iron-Catalyzed Hydrocarbon Oxidation
Dmitry V. Peryshkov, PhD, University of South Carolina
The aim of our research program is the development of ligands based on icosahedral boron clusters such as dicarbadecaborane C2B10H12. These polyhedral three-dimensional cages are attractive species for ligand design due to the unique combination of extreme steric hindrance and electron effects. The unusual icosahedral shape of these molecules provides the possibilty of formation of hemilabile vicinal B–H∙∙∙M bridging interactions when one of the boron vertices is metalated with the formation of the B–M bond. These interactions can stabilize low-coordinate metal fragments and provide an opportunity of potentially reversible functional group migration from the metal to the boron cage thus participating in the cooperative metal-ligand reactivity.
We have synthesized a novel carboranyl phosphinite ligand precursor 1,7-OP(i-Pr)2-m-carborane (POBOP-H) bearing two diisiosopropyl phoshpinite arms. Metalation of this ligand with rhodium led to B-H activation of one of the vertices and the formation the B–Rh and Rh-H bonds. The resulting (POBOP)Rh(H)(Cl)(PPh3) complex was converted to the square-planar (POBOP)Rh(PPh3) complex. The reaction of (POBOP)Rh(PPh3) and PhI led to the selective formation of the five-coordinate (POBOP)Rh(Ph)I complex featuring the highly strained B-Rh bond and close vicinal B-H∙∙∙Rh contact. The close contact between the metal center and the vicinal boron atom and the uniquely high strain of cage-metal bond in (POBOP)Rh(Ph)(I) resulted in the facile cascade reductive elimination/oxidative addition process leading to the aryl group transfer to the boron atom of the cage, subsequent B–H activation at the vicinal boron atom, followed by re-formation of the Rh(III) carboranyl hydride. This transformation demonstrated that the carboranyl unit can act not only as a supporting spectator ligand but also can be involved in cooperative ligand-metal reactivity.
The facile migration of a functional group to the boron cage and the activation of the vicinal B–H bond led us to a hypothesis that the carboranyl pincer framework could enforce the metal center to be in the proximity of both boron atoms of the cluster and lead to two B–H activation events with the formation of the three-membered (BB)>M metallacycle. Such a fragment would be an unprecedented all-boron inorganic analog of a transition metal benzyne complex containing the (CC)>M cycle. The carborane-based analogs of benzynes, carborynes, with two carbon atoms bonded to the single metal center has been reported; however, the boron-bonded analog with the (BB)>M metallacycle has not been known prior to our work. We successfully realized the synthesis of the first BB-carboryne metal complex in this PRF supported project.
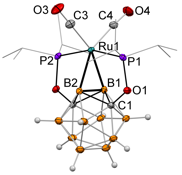
Figure 1. The molecular structure of the first (BB)-carboryne complex of ruthenium.
The reaction of the (POBOP-H) ligand precursor and [Ru(CO)3Cl2]2 resulted in the sequential double B–H activation leading to the formation of the first (BB)-carboryne complex of ruthenium, (POBBOP)Ru(CO)2 (POBBOP = 1,7-OP(i-Pr)3-2,6-dehydro-m-carborane). The indentity of the complex was confirmed by the array of methods including 1H, 13C, 31P, 11B, 11B{1H}, 11B-1H HSQC NMR spectroscopy, FTIR spectroscopy, and single-crystal X-ray diffraction. Importantly, the (POBBOP)Ru(CO)2 complex contained two highly strained B–Ru bonds with B–Ru distances of 2.174(3) and 2.221(3) and B–B–Ru angles 65.5(1)° and 68.4(1)°. The B–B bond length in the metallacycle exhibited a shortening to 1.720(4) relative to that in the (POBOP-H) ligand precursor (1.788(3) ). Theoretical calculations revealed that the (BB)>Ru metallacycle is formed by two bent B–Ru σ-bonds with the accompanying increase of the B–B bond order. Molecular orbitals of the (BB)>Ru fragment in the carboryne complex also bear the similarity to those of an olefin complex according to the Dewar–Chatt–Duncanson model.
The reactivity of the (BB)>Ru carboryne was probed by the reactions with the range of electrophiles. We found that the strained, electron-rich B–Ru bonds can themselves serve as the reaction centers in what can be described the oxidation addition of an electrophile to the metal-boron bond. Addition of terminal alkynes, inorganic acids, or halogens to the carboryne complex resulted in its selective transformation to the carboranyl complexes (POBOP)Ru(X)(CO)2. Only one metal–boron bond if the carboryne reacted with a substrate while another Ru–B bond remained intact.
PRF grant was critical in supporting the PI`s career development by funding the work on the use of icosahedral borane clusters as three-dimensional electron-donating ligands for transition metals. This project grew into the initially unanticipated area of chelating pincer complexes, resulting in the exciting synthesis of the first BB-carboryne metal complex. These results will serve as the foundation for the PI`s research program that aims to develop novel catalysts featuring ligand-metal cooperativity. With the aid of PRF financial support, six manuscripts have been published during two years. The graduate students supported by PRF fund obtained extensive training in synthetic organometallic chemistry, work with air-sensitive compounds, and related safety practices.