Reports: DNI655535-DNI6: Influence of Low Temperature Chemistry and Chemical Classes on MILD Combustion of Large Hydrocarbon Fuels
David Blunck, PhD, Oregon State University
1. Introduction
Moderate or Intense Low-Oxygen Dilution (MILD) combustion has the potential to significantly reduce pollutant emissions from applications requiring burning large hydrocarbon (i.e., liquid) fuels. Prior fundamental research has focused on MILD combustion of gaseous fuels. Consequently, it is not well understood how MILD combustion changes when different classes of large hydrocarbon fuels (e.g., aromatics, alkanes) are burned. With this motivation the overall objectives of this research are as follows: 1) Discover how MILD combustion changes when different classes of large hydrocarbons are burned, 2) Determine the effect of low temperature chemistry on the stability of MILD combustion burning large hydrocarbon fuels, and 3) Discover the chemical kinetic pathways which are responsible for changes in MILD combustion when low temperature chemistry is significant. The focus of the past year has been achieving objective 1.
2. Experimental and Computational Approaches
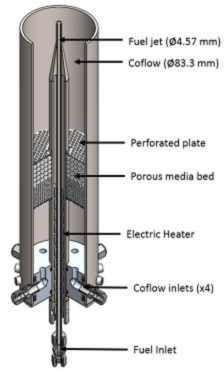
Both experiments and computations were performed to help achieve objective 1. MILD combustion was achieved using a jet in hot cross-flow burner (JHC), as shown in Figure 1.
Figure 1. Illustration of the jet in hot cross-flow burner.
The JHC burner consists of a central fuel jet surrounded by a coflow of hot combustion products. The elevated temperature and reduced oxygen concentrations of the coflow persist until nearly 100 mm above the central fuel jet. Thus, MILD or other high temperature conditions were created around the central fuel jet within about 10 cm of the burner exit.
Liquid fuels were injected into an electrically heated vaporizer where the fuel evaporated and was heated. The liftoff height of the flame was measured using chemiluminescence of OH* emissions. Visible images of the reaction zone were not always evident, or did not always agree with the flame location found using chemiluminescence.
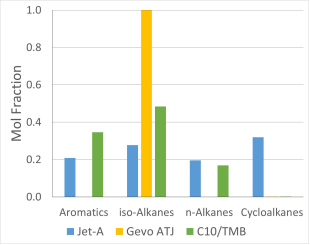
The liftoff height was measured as the following parameters were varied: fuel composition, the coflow temperature, the coflow oxygen concentration, and the Reynolds number of the central jet. The three fuels which were evaluated had different concentrations of the hydrocarbon classes, as illustrated in Figure 2. Jet-A had aromatics, iso- and n-alkanes and cycloalkanes, while Gevo ATJ had strictly iso-alkanes and C10/TMD had iso-alkanes and aromatics. The Reynolds number was changed from near 4,000 to 10,000.
Figure 2. Chemical classes of fuels tested.
Several analytical tools were used to gain insight into the trends in liftoff distance. Ignition delay and flame stretch calculations were performed to analyze the role of CH2O production in altering the flame anchoring location. Entrainment coefficients were used to further understand trends.
3. Results and Discussion
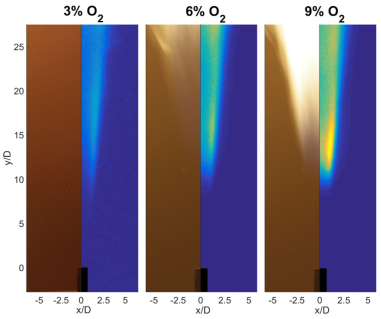
Visible and chemiluminescence images as ATJ fuel is burned in coflows with 3%, 6% and 9% oxygen concentrations are shown in Figure 3. For MILD conditions (3% O2) the reaction zone is not visible although emissions from OH* are present, verifying the presence of reactions. The location of visible and chemiluminescence emission becomes similar as the O2 concentration is increased to 9%.
Figure 3. Liftoff heights of the flame as the oxygen concentration of the coflow was varied from 3% to 9% (T = 1300 K).
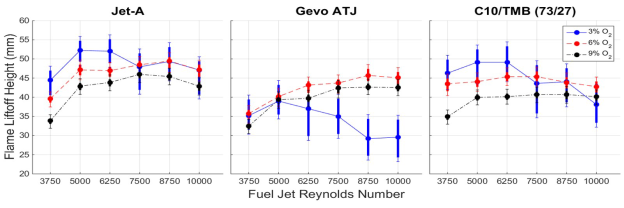
The liftoff height for flames burning the different fuels are reported in Figure 4 as the coflow oxygen concentration was varied from 3% to 9%. Three trends in the flame liftoff heights are evident.
Figure 4. Liftoff heights of the flame as the oxygen concentration of the coflow was varied from 3% to 9%. The temperature of the coflow as 1300K.
Trend 1. In coflows with 6% and 9% O2, a difference in liftoff heights is observed between the
three fuels. Jet-A produces flames with higher liftoff heights than Gevo ATJ flames,
while C10/TMB flames have the lowest liftoff heights.
Trend 2. All fuels burned with 6% and 9% O2 concentrations exhibit liftoff heights
that first increase with fuel jet Reynolds number and then decrease slightly or are constant at Reynolds numbers greater than 7,500. The liftoff heights of flames in 6% O2 are always higher
than flames in 9% O2.
Trend 3. Liftoff heights of flames in a coflow with 3% O2 were found to decrease as the
Reynolds number was increased above 6,250. Liftoff heights first increase as the coflow oxygen
concentration is increased from 3% to 6%, but they then decrease when changed from
6% to 9%.
The sensitivity of liftoff height to different fuels (i.e., trend 1) is attributed to changes in entrainment of the surrounding oxidizer into the central fuel jet. Flames burning C10/TMB have the highest entrainment coefficients at similar Reynolds numbers. This sensitivity results from differences in the fuel density.
The sensitivity of flame height to the Reynolds number for 6% and 9% O2 concentrations (i.e., trend 2) can be described by aspects of the premixed theory. The theory suggests that the flame height is inversely proportional to the flame speed, which would be higher for 9% O2 concentration.
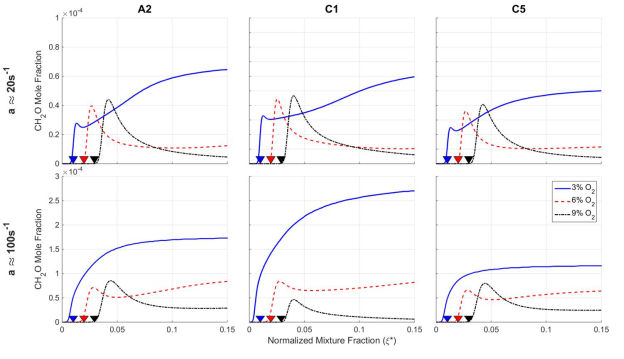
The counter-intuitive observation that the flame liftoff with 3% O2 concentration decreases with Reynolds number, and is less than or similar to when coflows have more oxygen (i.e., trend 3), is attributed to changes in ignition delay times and different sensitivities to flame stretch. The reaction zones are subject to increasing flame stretch as the Reynolds number is increased. Flame stretch increases production of CH2O, which decreases ignition delay times. Reactions with 3% O2 concentration tend to form more CH2O than conditions with 6% and 9% O2 concentration, hence decreasing flame liftoff. The sensitivity of CH2O production to flame stretch and oxygen concentration is illustrated in Figure 5 for opposed flame calculations.
Figure 5. Mass fraction of CH2O for different mixture fractions, oxygen concentrations, and flame stretch.
4. Future Work
Future work will consist of two primary tasks. First, identifying how the location of OH and CH2O within the reaction zone change for different fuel or coflow conditions. This will be achieved by applying planar laser induced fluorescence (PLIF). Second, elucidating the sensitivity of MILD combustion to low temperature chemistry. The burner will be modified to enable air dilution of the central fuel jet or injection of CH2O.