Reports: DNI553684-DNI5: Separating the Effects of Location and Substituent Identity on the Acidity of Microporous Materials
David Flaherty, PhD, University of Illinois, Urbana-Champaign
Transition metal phosphide (TMP) catalysts are selective and active towards C-O bond rupture during oxygenate hydrodeoxygenation (HDO); however, the way C-O bond rupture mechanisms and intrinsic barriers differ between transition metals and TMP are not well understood. We synthesized P-modified Ru(0001) catalysts via sequential PH3 dosage and performed CO and NH3 temperature programmed desorption (TPD) to investigate the effect of the addition of P atoms on the surface properties of a pristine Ru(0001).We also investigated the effects that the addition of P atoms has on the activation energies for bond rupture (e.g., C-O, C-C and C-H bonds) during carboxylic acid (e.g., formic acid (FA), acetic acid (AA), propionic acid (PA) and butyric acid (BA)) decomposition. These measurements were obtained from both pristine and P-modified Ru(0001) surfaces using a combination of TPR and reactive molecular beam scattering experiments (RMBS). These methods helped determine the changes in energy barriers and selectivities for C-O bond rupture under transient and steady-state conditions. These findings may provide useful information for the rational design of TMP catalysts to enhance C-O bond rupture, which will increase the efficiency of bio-mass conversion to platform chemicals.
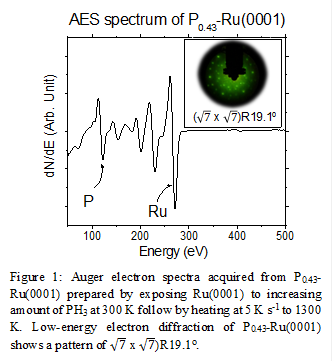
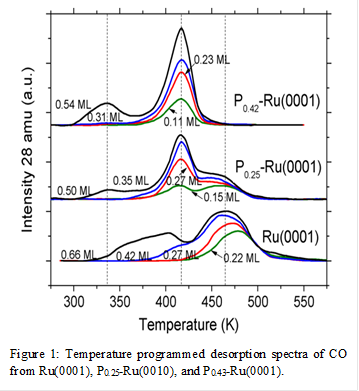
The Px-Ru(0001) catalysts (x is the coverage of P-atoms in monolayer equivalents) are prepared by dosing PH3 gas onto Ru(0001) followed by a 1300 K flash anneal. The resulting atomic P:Ru ratio (ranging from 0 to 0.4) is determined using the ratio of the Auger electron spectroscopy (AES) peak to peak intensities, while the crystallinity of P0.43-Ru(0001) is determined to have a low-energy electron diffraction (LEED) pattern of (√7 × √7)R19° shown in Figure 1. The LEED and AES results together suggest that P atoms form a crystalline structure the surface of which represents the Ru2P(111) facet. CO (shown in Figure 2) and NH3 TPD demonstrate that the addition of P-atoms to Ru(0001) decreases the binding energy of CO by 12 ± 2 kJ mol-1 at θ < 0.33 ML and that of NH3 by 11 ± 2 kJ mol-1 at θ < 0.1 ML compared to Ru(0001). These data suggest that P-atoms decrease the extent of electron exchange between Ru(0001) surface atoms and adsorbates.
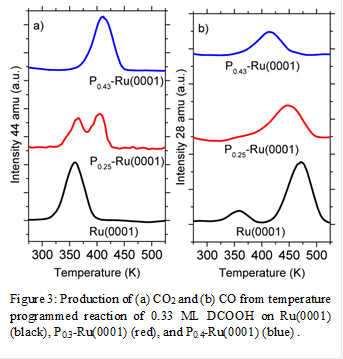
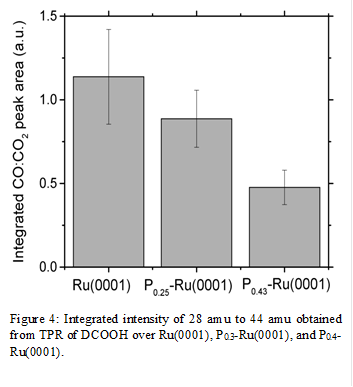
These findings are consistent with the changes in barriers for the decomposition of carboxylate surface intermediates. TPR spectra demonstrated that the addition of P atoms increase the temperature for formate(shown in Figure 3 and 4) and acetate decomposition, while decreasing the ratio of CO to CO2 production. This shows that P atoms increase activation barriers for C-O bond scission more than C-H and C-C bond scission. The increase in activation barrier can be attributed to the addition of P atoms decreasing the tendency for electrons to back donate from Ru atoms to the anti-bonding orbitals of the formate and acetate intermediate. Simple calculations show that the C-H bond activation barrier for formate decomposition to be 14 kJ mol-1 greater than on Ru(0001), while C-C bond activation barrier for acetate decomposition was 7 kJ mol-1 greater than on Ru(0001).
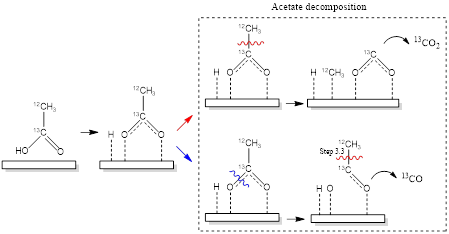
RMBS of formic acid on Ru(0001) and P0.4-Ru(0001) surfaces show that activation energies of C-O bond rupture and C-H bond rupture (measured from 500 - 700 K) are greater on P0.4-Ru(0001) than on Ru(0001). Steady-state production of CO to CO2 ratio, measured as a function of temperature, and that in TPR of FA, together demonstrate that the addition of P atoms to Ru(0001) favors C-O bond rupture more when compared to Ru(0001) at temperatures above ~ 475 K. Similarly, the addition of P atoms decreases the activation energies towards C-O, C-C, and C-H bond rupture during AA decomposition between temperatures of 450 – 600 K, while showing no significant difference towards C-O and C-C bond rupture at temperatures > 700 K. Lastly, based on TPR and RMBS data, we proposed elementary steps for FA and AA decomposition on pristine and P-modified Ru(0001). FA first adsorbs onto Ru(0001) and forms formate intermediate, which then decomposes either by dehydration, initiated by C-O bond rupture followed by C-H bond rupture to form CO or by dehydrogenation through a direct C-H bond rupture to form CO2. Similarly AA undergoes O-H dehydrogenation on the catalyst surface to form acetate, which then decomposes via C-C bond rupture to form CO2, CH3* or by C-O bond scission and subsequent C-C bond cleavage to form CO, H*, O* and CH3*; a portion of the residual C* forms recombinative CO which desorbs.
Scheme 1: Illustration of the mechanism for acetic acid decomposition on Ru(0001) and P-modified Ru(0001).
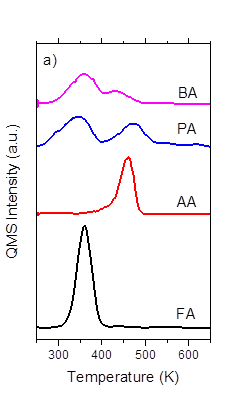
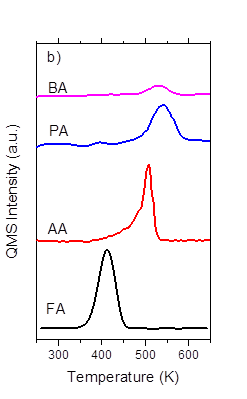
Most recently, we have found that the barriers for the decomposition of carboxylate intermediates are sensitive to the length of the alkyl substituents. The TPR results of PA and BA decomposition on Ru(0001) (shown in Figure 5a) demonstrated that the longer alkyl substituents decrease the CO2 production temperature, which suggests that longer alkyl chains decrease the C-C bond activation energy. These differences would be consistent with Bronsted-Evans-Polanyi predictions for the barrier of the elementary step for C-C bond rupture – in other words – the barriers for C-C bond rupture decrease with decreasing bond dissociation energies, which would be predicted by inductive effects. Further studies are underway to determine if the importance of other factors (e.g., adsorbate-adsorbate interactions via van der Waals interactions) that may depend on the size of alkyl substituent. .
Figure 5: Production of CO2 from TPR of formic acid, acetic acid,propionic acid, and butyric acid dosed at 250 K with a ramp rate of 3 K s-1 on a) Ru(0001) and b) P0.4-Ru(0001)..
In summary, the addition of P atoms to Ru(0001) increases the activation energy of C-O bond rupture and alters the metal surface-adsorbate interaction via electronic and/or geometric effects that stabilize carboxylate intermediates. These measurements show that the TMP catalysts may present high selectivities for hydrodeoxygenation catalysis due to changes in the inherent barriers for cleaving specific bonds in these reactants.