Reports: ND553999-ND5: Electron-Phonon Interactions in Atomically Monodispersed Gold Cluster/Graphene Oxide Nanocatalysts
Ramakrishna Guda, Western Michigan University
Overview
The overall goal of the project is to probe electron-phonon interactions in atomically precise-gold cluster/graphene oxide nanocatalysts. Quantum-sized metal clusters possess unique crystal structure where in the core-gold is passivated by a shell-gold with staple motifs. In continuation of our efforts to understand the electron-phonon interactions in quantum-sized metal clusters, following specific tasks are carried out.
(i) Temperature-dependent and solvent-dependent optical properties of bi-icosahedral Au25 clusters
(ii) Electron-phonon interactions in hexane-thiolate protected gold clusters
(iii) Temperature-dependent absorption and exciton relaxation dynamics in metal-doped Au25 and Ag25 clusters
Significant Results
(i) Temperature-dependent and solvent-dependent optical properties of bi-icosahedral Au25 clusters
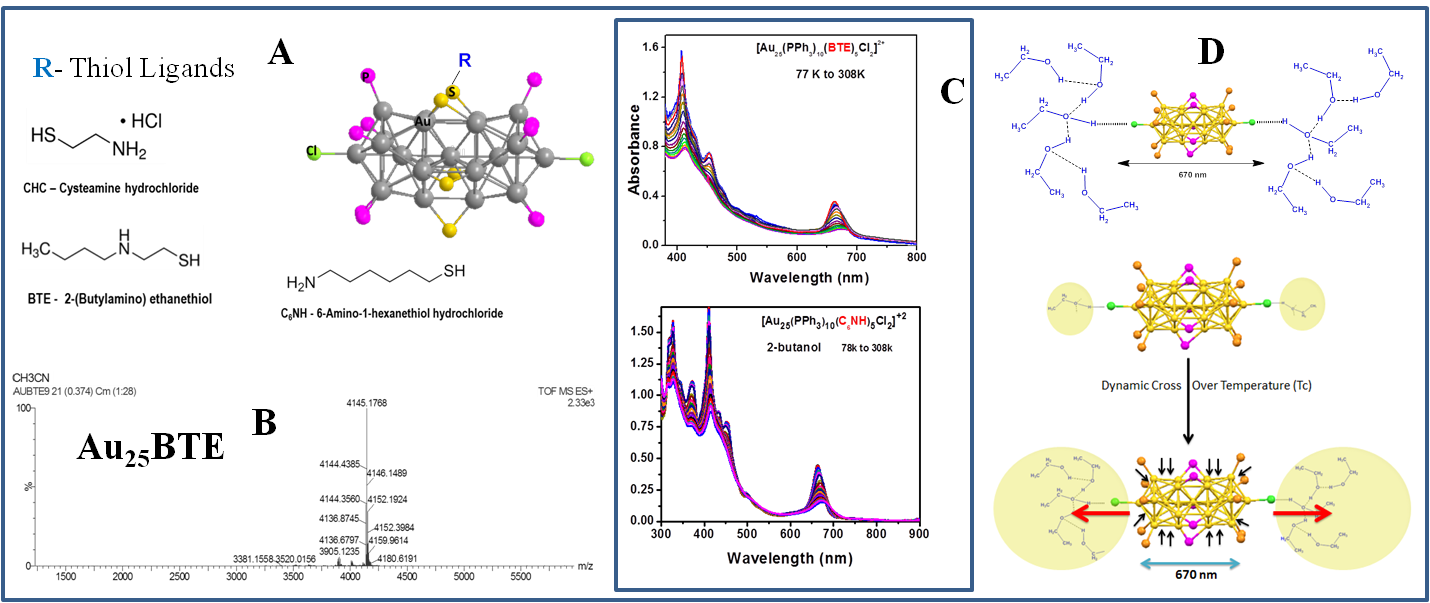
To develop gold cluster/graphene oxide nanocomposites, gold clusters with amine terminated ligands are synthesized that included water soluble spherical Au25 clusters passivated by captopril and glutathione ligands. Although these clusters possess amine groups at the end, it was difficult to probe their electron-phonon interactions as they are soluble in water and do not form good glass at low temperatures. For these reasons, we have synthesized bi-icosahedral Au25 (bi-Au25) clusters passivated with amine-terminated thiolated ligands. Several bi-Au25 clusters were made with different several amine-terminated thiols that included cysteamine hydrochloride (CHC), 2-(Butylamino) ethanethiol (BTE) and 6-Amino-1-hexanethiol hydrochloride (C6NH) (Figure 1A). The synthetic procedure was similar to what was reported for hexane-thiol capped bi-Au25 with some changes based on the ratio of reducing agent to gold phosphine and stirring conditions. Electrospray ionization – mass spectrometry was used to characterize the clusters and one such spectrum was shown for Au25-BTE in Figure 1B. Temperature-dependent absorption measurements were carried out for all clusters and the spectra of BTE and C6NH passivated bi-Au25 are shown in Figure 1C. The electron-phonon interactions were obtained from O’Donnell-Chen relationship and an energy of 20 ± 10 meV was obtained which is similar to hexanethiol passivated bi-Au25 clusters. The measurements have shown negligible influence of changing the ligand on electron-phonon interactions that should be good for their use as catalysts on the surface of graphene oxide. While carrying out measurements, an interesting solvent dependence of absorbance was observed at low temperatures. All the investigated bi-Au25 clusters in protic solvents have shown a zig-zag trend of absorption that was assigned to the hydrogen bonding of axial chlorine with hydroxyl group of protic solvents. This interaction becomes dominant only at low temperatures. This hydrogen bonding dominant after the dynamic cross-over temperature (Figure 2D) where the intramolecular hydrogen bonded ethanol alters the bi-icosahedron structure leading to splitting of energy levels. These amine-terminated clusters were bound to carboxy terminated graphene oxide via amide bond coupling reaction and their catalyst performance is being evaluated.
Figure 1. (A) Cartoon structures of bi-Au25 clusters capped with thiolated amines, (B) ESI-MS of [Au25(PPh3)10(BTE)5Cl2]2+ in acetonitrile, (C) Temperature-dependent absorption spectra of [Au25(PPh3)10(BTE)5Cl2]2+ and [Au25(PPh3)10(C6NH)5Cl2]2+ clusters and (D) hydrogen bonding formation of the solvent and cluster via the axial chlorine and the transition to high hydrogen bonding liquid at the dynamic cross-over temperature.
(ii) Electron-phonon interactions in hexane-thiolate protected gold clusters
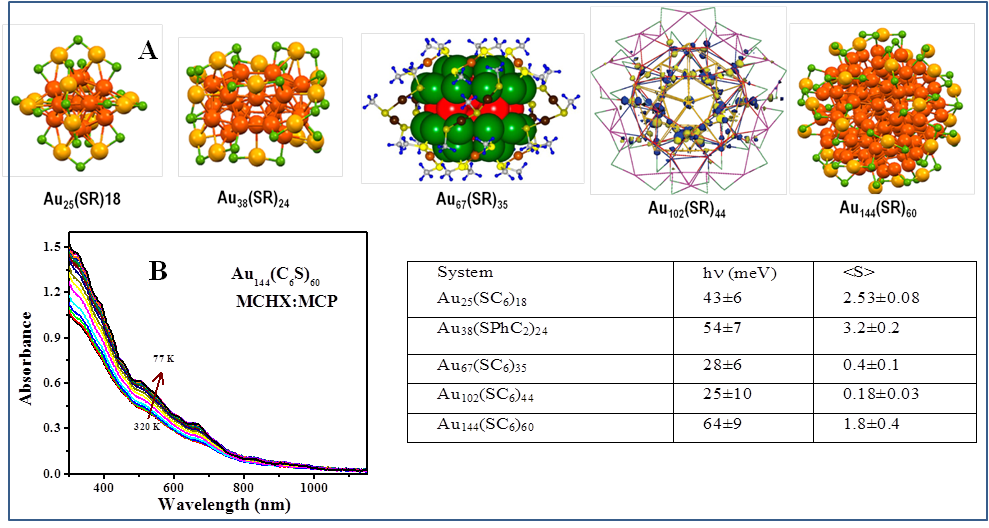
To understand if the size of cluster or the crystal structure impacts electron-phonon interactions in quantum-sized gold clusters, temperature-dependent absorption and ultrafast transient absorption measurements were carried out on hexane-thiolate protected gold clusters of varying sizes (25 to 144) (Figure 2A). Temperature-dependent absorption measurements were carried out on all clusters and representative absorption spectrum at different temperatures for Au144 is shown in the Figure 2B. A compilation of phonon energies obtained from fitting the energy vs temperature, oscillator strength vs temperature are provided in the table. It is observed from the table that the phonon energies are near 50 meV for smaller clusters and continued to decrease as the cluster size is increased to 67 and 102. However, energy of 64 meV was observed for Au144 cluster that points to the greater icosahedral symmetry of the cluster. In addition, ultrafast transient absorption measurements carried out for all these clusters show decreasing exciton lifetimes with an increase in the size of the cluster. The results are consistent with energy-gap law wherein the lifetime decreases with a decrease in the band gap. Also, the transient absorption measurements of Au144 cluster shows large number of bands in its excited spectra suggesting the presence of excitonic states for Au144 even though its band gap is quite small. The results show that both size and crystal structures of clusters play an important role on the electron-phonon interactions of quantum-sized gold clusters.
Figure 2. (A) Structures of investigated different sized hexane-thiolate protected gold clusters, (B) Temperature-dependent absorption of Au144 at different temperatures. Table summarizes the phonon energies and exciton-phonon coupling obtained from the analysis of temperature-dependent absorption.
(iii) Temperature-dependent optical properties of metal-doped Au25 and Ag25 clusters
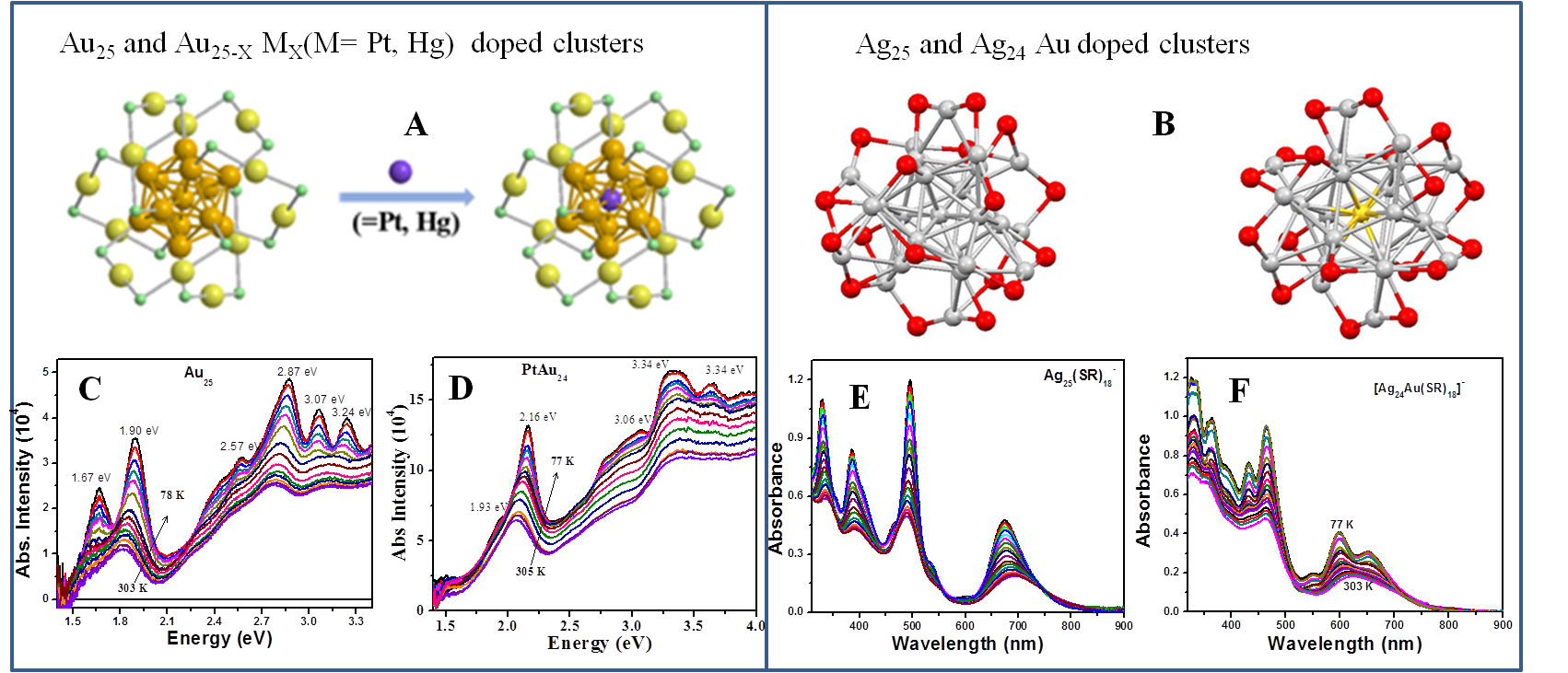
To understand how the electron-phonon interactions are altered with the doping of metal atom into clusters, metal-doped Au25 (Figure 2A) and Ag25 clusters (Figure 2B) were synthesized and their temperature-dependent optical properties were characterized. Metal-doped (Hg, Pt) Au25 clusters were synthesized using a protocol described previously and characterized with MALDI mass spectrometry. Temperature-dependent absorption measurements carried out on Au25 and PtAu24 are shown in Figure 2C and Figure 2D, respectively. Combined temperature-dependent absorption and ultrafast transient absorption measurements on metal-doped Au25 have shown that the metal atom doping alters the core-gold/shell-gold electronic coupling with increased phonon energies and modified exciton recombination. Temperature-dependent absorption measurements carried out on Ag25 (Figure 2D) and AuAg24 (Figure 2E) clusters have shown distinctly different absorption spectra at low temperatures with metal-doping and slower exciton recombination with gold doping. The results of metal-doped gold/silver clusters show that it influences the electron-phonon energies and band gaps that can have potential implications in their applications as catalysts.
Figure 3. Crystal structures of Au25 and Au24 (Pt, Hg) doped clusters (A) and Ag25 and AuAg24 clusters (B). Temperature-dependent absorption spectra of Au25 (C), PtAu24 (D), Ag25 (E) and AuAg24 (F). Phonon energies were obtained by fitting the energy gap vs. temperature plot to O’Donnell-Chen relationship.