Reports: DNI954621-DNI9: Advanced Characterization of Hydrate and Emulsion Formation in Flowing Systems for Flow Assurance Applications
Clint P. Aichele, Oklahoma State University
1. Introduction
Gas hydrates are non-stoichiometric, ice-like crystalline structures that are formed when lower weight hydrocarbons come in contact with water under suitable conditions. The key objective of this project is to investigate the extent and/or the influence of hydrate formation and dissociation on water-in-oil (w/o) emulsions stabilized using either particles of varying wettability or a surfactant both in the presence and in the absence of wax. In addition, the effect of hydrate forming guest molecule on Wax Appearance Temperature (WAT) was investigated. Furthermore, the effect of hydrate formation and dissociation on droplet size of the water-in-oil emulsions stabilized using either surfactants or solid particles were quantified.
2. Materials and Methods
Deionized water (18.2 mΩ cm-1) was used to form the internal phase. The continuous oil phase consisted of light mineral oil, and either cyclopentane or iso-octane. Cyclopentane was used as the hydrate-forming component. Sorbitan monooleate (Span 80), an oil soluble, non-ionic surfactant was used to stabilize the water-in-oil emulsions. Solid stabilized emulsions were prepared using solid particles that were purchased from Evonik Inc.
2.1 Experimental protocol
For the investigations on the effect of hydrate formation on emulsion stability, the entire water-in-oil emulsion sample, which was prepared using the homogenizer, was placed in the chiller (HAAKE N8-C41 chiller). Once the sample temperature reaches 2 0C to 3 0C, ice crystals were added to the sample to aid nucleation. Then, sample was sheared using the homogenizer at a shear rate of 2800 rpm for 3 hours. After 3 hours, the water-in-oil emulsion, which experienced hydrate formation, was taken out of the chiller and was allowed to reach the room temperature. A sample of the residual emulsion was observed under the microscope to detect the effect of hydrate formation and dissociation on the droplet size of the water-in-oil emulsion. The photomicrographs, which were taken at the 0th hour and after hydrate dissociation, were analyzed using Image J to quantify the droplet size distribution.
3. Results and Discussion
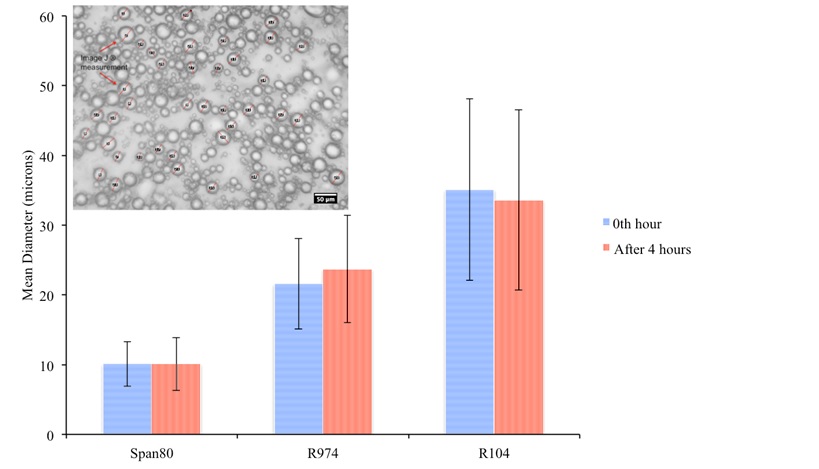
From Figure 1, it can be seen that the droplet size increases with an increase in the hydrophobicity of the solid particle (R104 is more hydrophobic than R974).
Figure 1: Effect of stabilizer on droplet size of w/o in emulsions at 40 vol.% water cut
3.1 Effect of hydrate formation and dissociation on emulsion stability
3.1.1 Microscopic characterization
Table 1 shows that the droplet size of the surfactant stabilized emulsion increased by approximately more than 85% after hydrate dissociation unlike solid stabilized emulsion, which did not exhibit a significant change in the droplet size of the residual emulsion after hydrate dissociation.
Water-in-oil emulsion with 40 vol.% water cut | ||
Stabilizers | Mean droplet size (μm) | |
Before Hydrate formation | After Hydrate Dissociation (From residual emulsion) | |
Span 80 | 10.1±3.2 | 18.9±14.9 |
R974 | 21.6±6.5 | 16.6±7.6 |
R104 | 35.1±13.0 | 29.2±10.4 |
Water-in-oil emulsion with 20 Vol.% water cut | ||
Span 80 | 4.4±2.1 | 8.9±3.5 |
R974 | 11.0±2.9 | 11.2±3.3 |
R104 | 17.9±4.5 | 15.1±4.5 |
Table 1: Effect of hydrate formation and dissociation on mean droplet size of w/o emulsions
3.2.2.Macroscopic characterization
Figure 2 shows the effect of hydrate formation and dissociation on the stability of water-in-oil emulsions with 20 vol.% water cut. Hydrate formation and dissociation causes destabilization of water-in-oil emulsions stabilized using a surfactant or highly hydrophobic solid particles unlike moderately hydrophobic solid particles.
Figure 2: Effect of hydrate formation and dissociation on stability of water-in-oil emulsions with 20 Vol.% water cut.
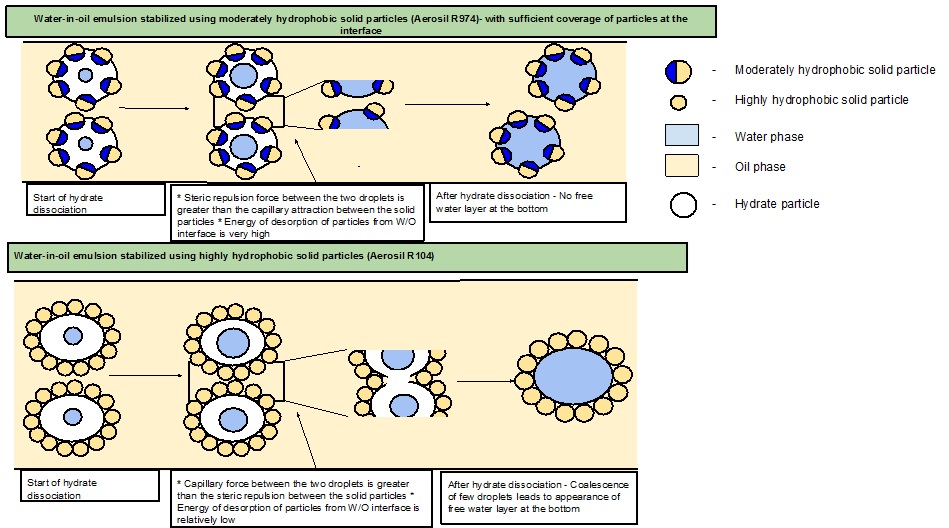
Figure 3 provides the schematic of the hypothesized mechanism explaining the phenomenon for the observed difference in stability of solid stabilized w/o emulsions upon hydrate dissociation.
Figure 3: The schematic of the hypothesized mechanism explaining the phenomenon for the observed difference in stability of solid stabilized w/o emulsions.
Table 2 shows the effect of cyclopentane on WAT. The presence of cyclopentane had a significant effect on WAT.
Systems | WAT (°C) |
Light mineral oil + wax + H1 + water | 26.5 |
Light mineral oil + Cyclopentane + wax + H1 + water | 20.7 |
Light mineral oil + Cyclopentane + wax + H2 + water | 23 |
Light mineral oil + Cyclopentane + wax + H3 + water | 22.2 |
Table 2: Effect of cyclopentane on WAT
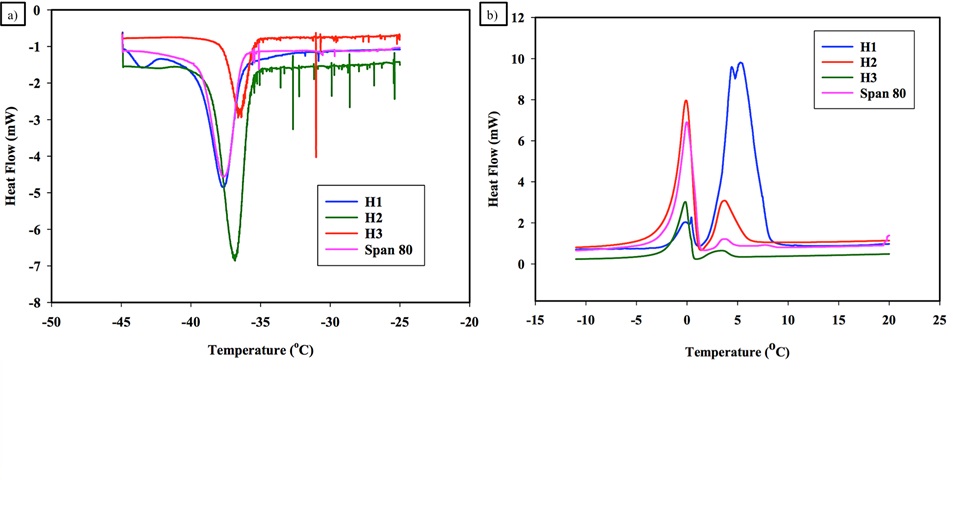
Figure 4 shows the cooling and heating cycles in a DSC for hydrate forming water-in-oil emulsions stabilized using either solid particles of varying hydrophobicity (least hydrophobic (H1), intermediate hydrophobicity (H2), highly hydrophobic H3) or a surfactant. It can be concluded that the amount of hydrate formation decreased with an increase in hydrophobicity of solid particles. Furthermore, it can be seen that solid particles promote hydrate formation to a greater extent than a surfactant.
Figure 4: Effect of stabilizers on hydrate formation
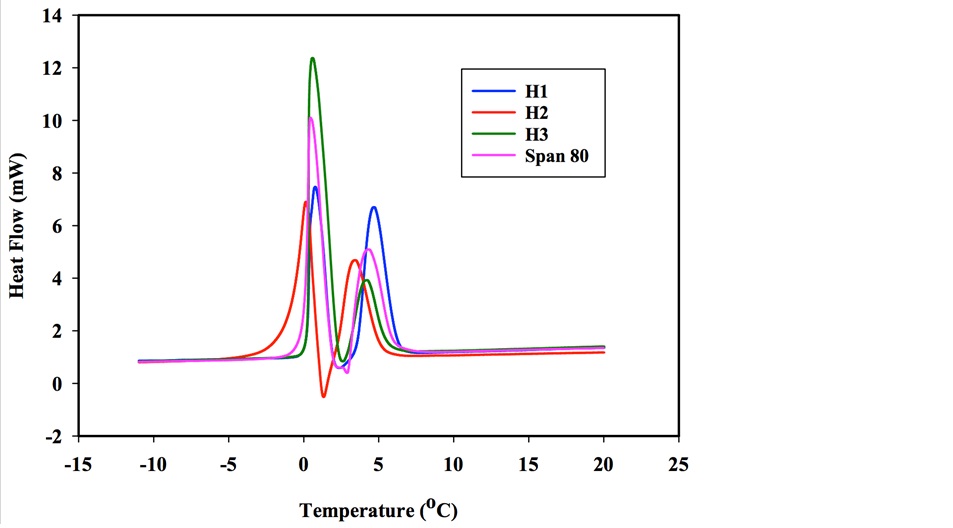
The effect of wax on the extent of hydrate formation in water-in-oil emulsions can be seen from Figure 5. The presence of wax enhanced hydrate formation in water-in-oil emulsions stabilized using intermediate hydrophobic particles, highly hydrophobic solid particles, and a surfactant unlike water-in-oil emulsions stabilized using least hydrophobic particles.
Figure 5: The effect of wax on hydrate forming w/o emulsions.
4. Conclusions
The presence of moderately hydrophobic particles resists destabilization of w/o emulsions after hydrate dissociation unlike w/o emulsions stabilized using highly hydrophobic particles or a surfactant. The presence of cyclopentane reduces the WAT. In general wax promotes hydrate formation. Hydrate formation is greater for w/o emulsions stabilized using least hydrophobic particles as compared to highly hydrophobic particles.
5. Future Work
A re-designed flow loop was recently installed at an OSU facility that can safely accommodate the use of flammable liquids and gases (see Figure 5). The flow loop will be used to investigate emulsion and hydrate behavior in flowing conditions.
Figure 6. Re-designed flow loop for emulsion and hydrate characterization.
6. Impact
This project has positively impacted PI-Aichele’s career by providing him with the opportunity to explore a fascinating area of hydrates and emulsions. The project has facilitated novel research and supported both graduate and undergraduate students and one postdoctoral scholar. This project has enabled his group to contribute to the scientific community through the publication of two manuscripts, and at least two more publications are expected.