Reports: DNI554107-DNI5: Naphthenic Acid Ketonization Mechanism over Zeolites
Steven Crossley, University of Oklahoma
A variety of carboxylic acids are present in petroleum derived streams in the form of naphthenic acids. Carboxylic acid impurities are present in a variety of petroleum derived streams that are commonly upgraded with zeolite catalysts (e.g. catalytic cracking, hydrocracking). Interestingly, little is known regarding the mechanism for conversion of such carboxylic acids over Brønsted zeolite catalysts. We use a combination of surface spectroscopy and reaction kinetics to identify the reaction mechanism and intermediates present when converting carboxylic acids over zeolites. To summarize our findings during the past year, in-situ surface IR experiments have verified the presence of stable surface acyl species at moderate conditions, confirming our hypothesis that dehydration is not a rate determining step. By measuring reaction kinetics, we have also shown that the constant for catalyst deactivation as a function of time is significantly reduced by the presence of water, which we attributed to interaction of water with intermediate ketene species. This has important implications in refining, as naphthenic acids capable of producing ketenes will lead to rapid coke formation, but this can be mitigated by introducing water, which is already present in some refining processes such as fluid catalytic cracking. The remainder of this report will summarize our findings based on reaction kinetics that have recently been published in Journal of Catalysis. Acetic acid is used as a model carboxylic acid compound in the experiments and discussion below.
Reaction kinetics and identification of rate-determining step
Tests were conducted to ensure that the kinetic data is not limited by mass transfer. The reaction rate at 310°C as a function of Helium carrier gas flow rate is used to verify these conditions. At low flow rates, the rate increases with increasing carrier gas flow, indicating that the rate is controlled by external mass transport to the catalyst surface. This effect disappears above 100 ml/min of gas flow suggesting the reaction is devoid of external diffusion corruptions above this flow rate. All kinetic experiments were therefore conducted with a carrier gas flowrate of 125 ml/min. This includes the experiments reporting reaction orders vs. TOS as described in the previous section.
The necessity of Brønsted acid sites for this reaction to proceed was confirmed by passing acetic acid over silicalite-1 at 300°C, with no measurable conversion observed over silicalite-1. This was further confirmed by Na exchange of the HZSM-5 The activity dropped proportional to the concentration of Brønsted sites passivated by Na, with a constant TOF of 1.03±0.09 min-1 per Brønsted site over all of the catalysts tested. This confirms that the measured rates are not limited by internal or external mass transfer under these conditions. This result also demonstrates that any Lewis acidity introduced by the incorporation of Na cations does not accelerate the rate-determining step.
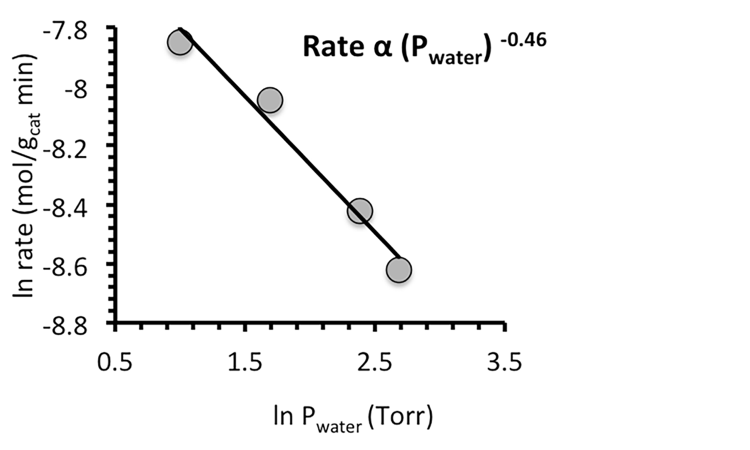
Figure 1:- Natural logarithm of the rate of reaction vs. acetic acid (top) and water (bottom) concentration over CBV8014 at 300°C, 0.025 g catalyst, with rates extrapolated to 0 min TOS.
By varying the concentration of acetic acid fed, we observe that the order of the ketonization reaction is greater than one. Rates were found by extrapolating the conversion to initial TOS values to eliminate effects of catalyst deactivation. The fact that the reaction appears to be greater than 1st order as shown in Figure 1 implies a rate-determining step involving two acid-derived species. The reason for an observed order with respect to acetic acid less than two could be due to coverage of acetic acid and acid derived surface species. This behavior is very similar to that observed when measuring the apparent reaction order for coupling of acids over reducible oxides, where the second order coupling exhibits a fractional apparent order.
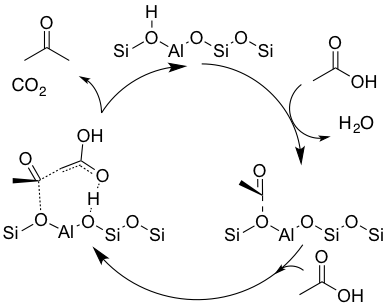
Combining the observance of water evolution at lower temperatures than acetone and CO2 along with the observance of acyl surface species at 150°C via infrared studies implies that the dehydration of an acetic acid molecule to form a surface acylium species is not the rate-determining step. This instead suggests that an intermediate formed between an acylium ion and an activated carboxylic acid can couple and subsequently decompose to yield CO2 and acetone as shown in Figure 2.
Figure 2:- Proposed reaction pathway for carboxylic acid coupling over a Brønsted zeolite.
The kinetic order greater than one further supports a second order rate-limiting step. This second order dependence that is manifested as an apparent 1.6 order rate dependence on acetic acid partial pressure can be explained by examination of a rate law derived assuming that an adsorbed acid and an adsorbed acyl interact during the rate-determining step to form acetone.
An expression
highlighting the second order dependence of this rate-determining step is
derived in the supplemental information, resulting in the following equation:
![]() (1)
(1)
Several scenarios depicting variants of most abundant surface intermediates (MASI) are highlighted in the supplemental information. One can clearly see by inspection of (1) that a fractional order of 1.6 with respect acetic acid can be attributed to contributions to the rate equation by a combination of surface acetic acid, acetic acid/acyl complex, and surface acyl groups (corresponding to the three terms in the denominator that increase with the partial pressure of acetic acid).
Evaluation of this expression and the reaction orders shows that the surface is populated by a significant portion of acyl species under reaction conditions. Further analysis of the transition states reveals the absence of a primary kinetic isotope effect upon substitution of H by D in C2 acid probe molecules. This demonstrates that the transition state involves the formation of a C-C bond in figure 2, while primary cleavage or formation of C-H bonds do not directly participate in the transition state.
Figure 3:- TOF values for acetic acid, and it's deuterated counterparts in the OD position (D1) and fully deuterated (D4).