Reports: UNI154721-UNI1: Chiral Diaziridines as Synthetic Building Blocks for the Systematic Synthesis of Nitrogen-Containing Heterocycles
Gustavo Moura-Letts, PHD, Rowan University
Diaziridines are an important member of a group of organic building blocks that contain two electronegative N atoms in a strained three-membered ring arrangement. Due to the inherent strain in the three-membered ring and the presence of an adjacent nitrogen atom, the nitrogen stereocenter of diaziridines exhibits remarkable configurational stability (inversion barrier 32 kcal/mol). Thus, their application as a template for the production of more complex heterocycles has great potential.
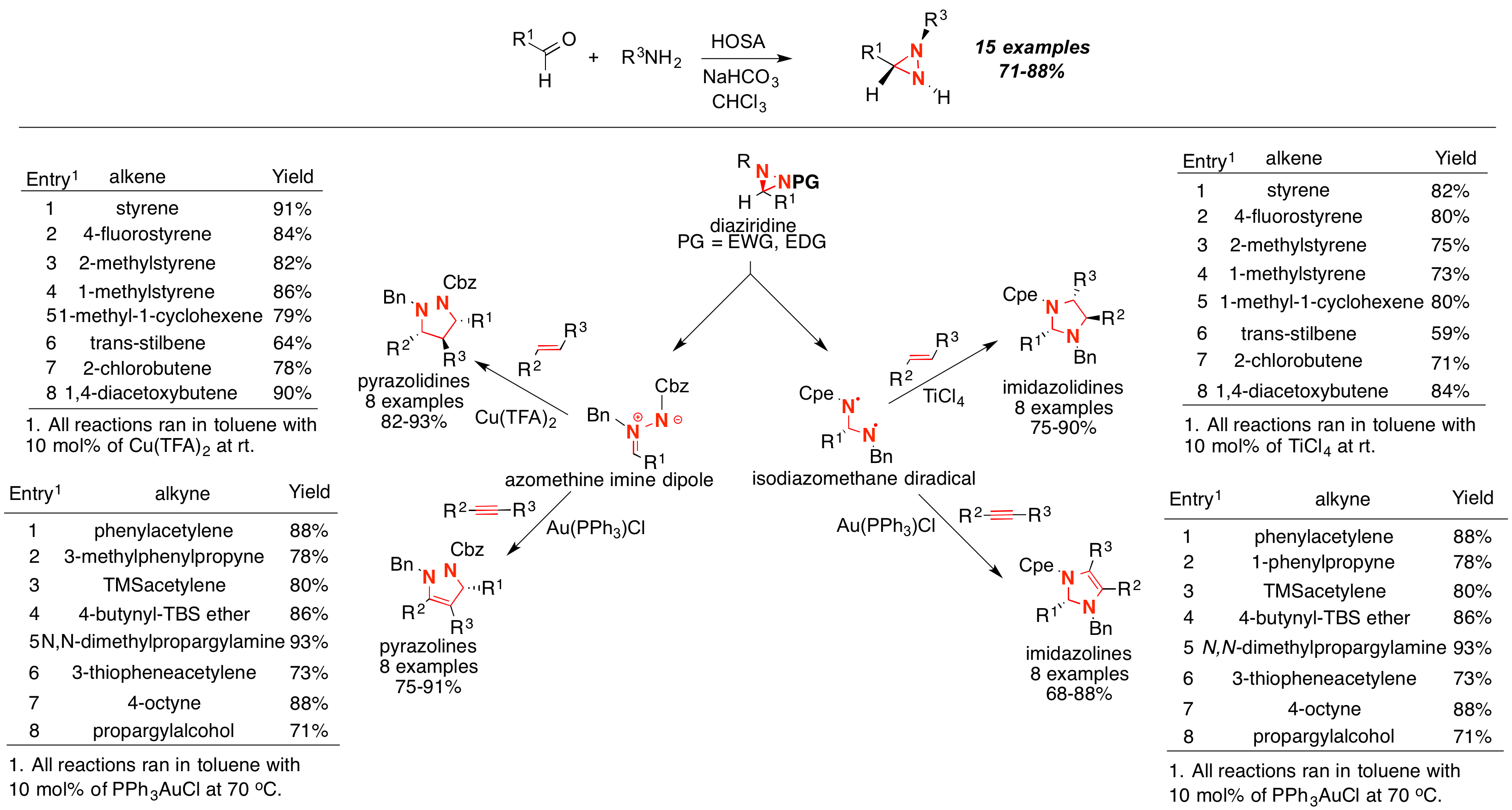
In our laboratory, we have established a strong research platform for the development of synthetic methods to produce N,N-containing heterocycles. We discovered that aldehydes and amines react under mild basic conditions in the presence of HOSA to form diaziridines in high yields and diastereoselectivities (Scheme 1). Enones, enals and anilines can also be productive substrates under mild heating in water. As proposed in this grant, we were interested in establishing whether these diaziridines could be used as chemical synthons for the synthesis of five membered ring N,N-containing heterocycles. We hypothesized that the difference in electronic properties of the groups around the N atoms in the diaziridine substrate would control the chemoselectivity of the cycloaddition. Thus, groups of opposing electronic properties (Electron donating groups (EDG)/electron withdrawing groups (EWG)) would undergo cycloaddition across the C-N bond, and groups of similar electronic properties (EDG/EDG) would react across the N-N bond.
We also found that diaziridines can be easily protected by protecting groups with a variety of electronic and steric properties in very high yields (Bn, PMB, Cbz, Boc, Ac, TBS). These compounds were then exposed to optimized cycloaddition conditions based on their protecting groups. When the electronic relationship between each group bound to the N atoms was different (EDG/EWG = Bn/Cbz), we were able to synthesize a large variety of pyrazolines (AuPPh3Cl for dipolar cycloaddition with alkynes) and pyrazolidines (Cu(TFA)2 for dipolar cycloaddition with alkenes) in very good yields and regioselectivities. Based on our observations, we proposed an azomethine imine dipole intermediate for the formation of the respective pyrazolines and pyrazolidines. Moreover, when the relationship among the protecting groups bound to N was matched (EDG/EDG = Bn/PMB), we found complete reversal of the chemoselectivity for the formation of imidazolines (AuPPh3Cl for dipolar cycloaddition with alkynes) and imidazolidines (TiCl4 for dipolar cycloaddition with alkenes) in very good yields and regioselectivities. Accordingly, we proposed an isodiazomethine diradical intermediate for the formation of the respective imidazolines and imidazolidines (Scheme 1). These results demonstrated that heterocycles like diaziridines undergo cycloaddition reactions with high yields and stereoselectivities. Moreover, we found that high levels of chemocontrol could be achieved by changing the electronic relationship between the groups bound to the N atoms. We are in the process of preparing a few manuscripts for publication that will describe these results. This success has propelled an array of other research platforms where we are investigating other cycloaddition and rearrangements for the efficient synthesis of more complex N,N-containing heterocycles.
Scheme 1. Synthesis and cycloaddition reactions of diaziridines.
The success of diaziridine project has allowed our research group to focus on developing methods for the synthesis of N-containing molecular scaffolds. These efforts have allowed publishing seven manuscripts involving the study of such synthetic transformations.
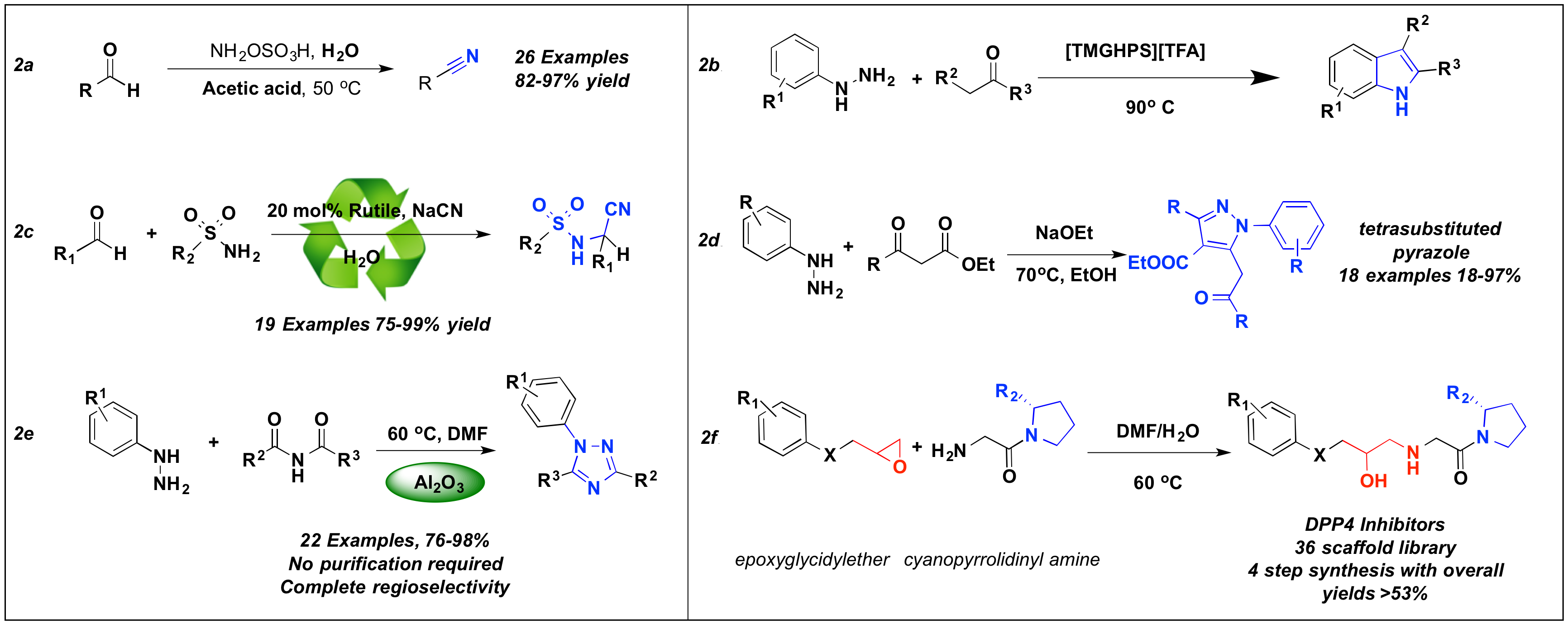
The initial study on the synthesis and chemical reactivity of diaziridines showed us that aldehydes in the absence of an amine, react with HOSA under slightly acidic conditions to produce nitriles with complete conversion and excellent yields. The scope for this method expanded to a large variety of aliphatic, aromatic, and chiral aldehydes with similar outcomes (Scheme 2a).
The interest in obtaining relevant N-containing heterocycles prompted us to search for mild methods for the synthesis of substituted indoles. We found that protonic Brønsted acid ionic liquids allow for the synthesis of these scaffolds with great conversion and yields. The results allowed us to recover and recycle the ionic liquid without eroding the productivity of the reaction (Scheme 2b).
The development of green reaction conditions for the synthesis of N-containing nitrogen scaffolds is a major focus in the field of organic chemistry. We were interested in the efficient synthesis of aminonitriles and we found that the reaction of aldehydes and ketones with amines with NaCN and TiO2 produced the respective aminonitrile in great conversions and yields. The TiO2 promoter was also recovered and recycled without eroding the productivity of the reaction (Scheme 2c).
The synthesis of N,N-containing heterocycles is the major research platform in our laboratory. This interest expanded beyond their synthesis through the cycloaddition of diaziridines. This study focused on the reaction of substituted hydrazines and ketoesters under basic conditions for the synthesis of tetrasubstituted pyrazoles. This unique reaction had a pronounced scope for a large number of hydrazines but was limited to ethylacetoacetate. Other ketoesters were only able to provided the more expected pyrazolone scaffolds (Scheme 2d).
Scheme 2. Published efforts for the synthesis of N-containing scaffolds
1,2,4-Triazoles were another heterocyclic scaffold of high relevance in our efforts to develop novel synthetic approaches to heterocycles. We found that aromatic hydrazines and imides in the presence of Al2O3 provide the respective N-aryl-1,2,4-triazoles in great yields without need for purification. The scope for this reaction extended to symmetrical and unsymmetrical imides (Scheme 2e).
Lastly, we were also interested in developing more efficient methods for the synthesis of cyanopyrrolidinyl amino alcohols. This study found that under mix-solvent conditions we could selectively achieve the epoxide-opening reaction with primary cyanopyrrodinylamines to provide the expected products (Scheme 2f).
The support form the ACS Petroleum Research Fund has allowed us to generate a large amount of research in the field on N-containing molecular scaffolds. This support has also created multiple novel research platforms from the reported efforts that we hope would be suitable for further ACS PRF funding.