Reports: UR153237-UR1: Investigation of a Stereoselective Tandem Inverse-Demand Hetero-Diels-Alder/Tin-Free Radical Process for the Synthesis of Highly Substituted Heterocycles
Jake R. Zimmerman, PhD, Ohio Northern University
My research group has benefited tremendously from the support of the ACS-PRF-UR grant. It has allowed me to fund six summer research students over the three years of the grant and purchase a variety of expendables that were needed for our projects. Four of my research students have graduated over the past three years and are now attending graduate school. Two other students are now in their senior year at ONU and both plan to attend graduate school in chemistry. Because of this support, my group has published two papers and are now very close to another submission. Below is a summary of my group's progress over the past year (9/1/15 – 8/31/16).
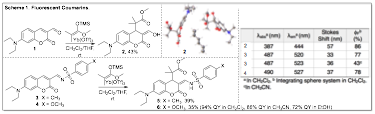
My group spent the final year of this grant working on a project that involved synthesizing a new class of highly fluorescent chromone and coumarin derivatives. In the first two years of the grant period, we discovered an inverse-demand hetero-Diels-Alder (IDHDA) reaction using silylenol ethers and 3-formylchromones. Interestingly, it was discovered that these Diels-Alder products were highly fluorescent. We then synthesized a small library of these compounds and studied their photo-physical properties. Simple irradiation of these compounds using a basic long-wave UV lamp (~365 nm) resulted in blue to green fluorescence. This past year, we focused on investigating a new class of coumarins using our IDHDA reaction. Some of the results are shown below in Scheme 1. Compound 1 was reacted with a silylenol ether in the presence of Yb(OTf)3 to give a similar Diels-Alder adduct that opens up to the enol upon quenching on silica. Enol 2 exhibits a very high quantum yield (86%) in CH2Cl2. We were also able to convert 1 into the sulfonamide Schiff base derivatives 3 and 4. Interestingly, the Diels-Alder product 6 showed very impressive quantum yields in a variety of solvents as shown below. Although coumarins have been extensively studied over the years, to the best of our knowledge these newly synthesized compounds have not been reported in the literature. We are currently, synthesizing more of these derivatives in order to study their spectroscopic properties. Our goal is to publish this work in the future.
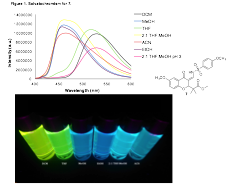
My research group also has been investigating an interesting property of these new fluorophores, which is called solvatochromism. This phenomenon is the change of emission wavelength (color) by varying the polarity of the solvent the fluorophore is dissolved in. For instance, in more nonpolar solvents such as CH2Cl2 the emission is ~530 nm (green), however, in more polar solvents such as methanol, the emission shifts to a shorter wavelength ~455 nm (blue) (all spectra taken at 5 mM). The emission sprectra for 7 in various solvents is shown below in Figure 1.
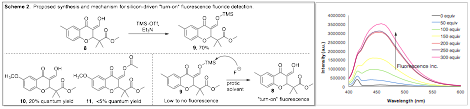
Another ongoing project is the expansion of our fluoride detection that was reported in last year's narrative. We have shown that our compounds can detect fluoride anion via a ~40 nm blue shift in the emission spectrum when fluoride is present with our fluorophore. We propose this happens due to a hydrogen-bonding mechanism of fluoride with our compound. Because of the mechanism, we can only detect fluoride in more nonpolar, aprotic organic solvents. One goal we've had for this past year was to detect fluoride in a protic solvent such as methanol. In order to do this, we fundamentally changed the fluoride detection mechanism from hydrogen-bonding to a chemical reaction. Scheme 2 outlines the work we carried out. It was discovered that if the enol proton was replaced with a TMS group, the fluorescence was quenched. Therefore, we protected the enol 8 with TMS and then added varying equivalents of fluoride to see if our new sensor would work as a 'turn-on' fluorescent detector in the presence of fluoride. It was discovered that our new chemical sensor (9) does work, however, it takes a very large excess of fluoride for the fluorescence to increase significantly. Therefore, this project is still very much in its infancy and we plan to continue studying this in the future.
In conclusion, we will continue to investigate the synthesis, solvatochromic properties, and sensing properties of these new fluorescent chromones and courmarins.